
Introduction
Influenza, commonly known as the flu, is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness and can lead to hospitalization and even death.[1] The aim of this guide is to provide healthcare professionals with a comprehensive overview of the diagnosis and management of influenza.
Codes
- ICPC-2 Code: R80 Influenza
- ICD-10 Code: J11.1 Influenza with other respiratory manifestations, virus not identified[2]
Symptoms
- Fever: Usually high, often above 100.4°F (38°C)
- Cough: Dry or productive cough
- Sore throat: Irritation or pain in the throat
- Runny or stuffy nose: Nasal congestion or discharge
- Muscle or body aches: Generalized body pain or discomfort
- Headache: Pain or pressure in the head
- Fatigue: Feeling tired or exhausted
- Chills: Feeling cold or shivering
- Nausea or vomiting: Some individuals may experience gastrointestinal symptoms
- Diarrhea: Some individuals may experience gastrointestinal symptoms[3]
Causes
- Influenza viruses: Influenza A, B, and C viruses are responsible for causing influenza. Influenza A and B viruses are the most common causes of seasonal flu outbreaks.[4]
Diagnostic Steps
Medical History
- Gather information about the patient’s symptoms, including the onset, duration, and severity.
- Ask about any recent exposure to individuals with confirmed or suspected influenza.
- Inquire about any underlying medical conditions or risk factors that may increase the patient’s susceptibility to influenza complications.
- Assess the patient’s vaccination history, including the receipt of the annual influenza vaccine.[5]
Physical Examination
- Measure the patient’s temperature to assess for fever.
- Examine the patient’s respiratory system for signs of congestion, such as nasal discharge or cough.
- Assess the patient’s throat for redness or inflammation.
- Palpate the patient’s muscles and joints for tenderness or pain.
- Evaluate the patient’s general appearance for signs of fatigue or malaise.[6]
Laboratory Tests
- Rapid influenza diagnostic tests (RIDTs): These tests detect influenza viral antigens in respiratory specimens. They provide quick results within 15-30 minutes but may have lower sensitivity compared to other tests.
- Reverse transcription-polymerase chain reaction (RT-PCR): This test detects the presence of influenza viral RNA in respiratory specimens. It is highly sensitive and specific and can differentiate between influenza A and B viruses.
- Viral culture: This test involves growing influenza viruses in a laboratory to confirm the presence of the virus and determine its type and subtype. It is more time-consuming and may take several days to yield results.[7]
Diagnostic Imaging
- Chest X-ray: This imaging modality may be used to evaluate patients with severe respiratory symptoms or suspected complications, such as pneumonia.[8]
Other Tests
- Complete blood count (CBC): This test may reveal leukopenia or lymphopenia, which can be associated with influenza infection.
- C-reactive protein (CRP): Elevated CRP levels may indicate inflammation and can help differentiate influenza from other respiratory infections.
- Nasopharyngeal swab for respiratory pathogen panel: This test can detect multiple respiratory pathogens, including influenza viruses, in a single sample.[9]
Follow-up and Patient Education
- Advise the patient to rest and stay hydrated.
- Educate the patient about the importance of hand hygiene and respiratory etiquette to prevent the spread of influenza.
- Discuss the potential complications of influenza and when to seek medical attention.
- Encourage the patient to receive the annual influenza vaccine to reduce the risk of future infections.[10]
Possible Interventions
Traditional Interventions
Medications:
Top 5 drugs for Influenza:
- Oseltamivir (Tamiflu):
- Cost: $50-$100 for a 10-pill pack.
- Contraindications: Hypersensitivity to oseltamivir or any component of the formulation.
- Side effects: Nausea, vomiting, headache.
- Severe side effects: Neuropsychiatric events, including self-injury and delirium.
- Drug interactions: None of clinical significance.
- Warning: Start treatment within 48 hours of symptom onset for optimal efficacy.
- Zanamivir (Relenza):
- Cost: $50-$100 for a 20-dose inhaler.
- Contraindications: Hypersensitivity to zanamivir or any component of the formulation.
- Side effects: Bronchospasm, cough, headache.
- Severe side effects: None reported.
- Drug interactions: None of clinical significance.
- Warning: Not recommended for patients with underlying respiratory conditions, such as asthma or chronic obstructive pulmonary disease.
- Peramivir (Rapivab):
- Cost: $200-$300 per dose.
- Contraindications: Hypersensitivity to peramivir or any component of the formulation.
- Side effects: Diarrhea, nausea, vomiting.
- Severe side effects: None reported.
- Drug interactions: None of clinical significance.
- Warning: Administered intravenously and typically reserved for hospitalized patients.
- Baloxavir marboxil (Xofluza):
- Cost: $150-$200 for a single-dose pack.
- Contraindications: Hypersensitivity to baloxavir marboxil or any component of the formulation.
- Side effects: Diarrhea, bronchitis, nausea.
- Severe side effects: None reported.
- Drug interactions: None of clinical significance.
- Warning: Start treatment within 48 hours of symptom onset for optimal efficacy.
- Amantadine (Symmetrel):
- Cost: $10-$20 for a 30-day supply.
- Contraindications: Hypersensitivity to amantadine or any component of the formulation.
- Side effects: Dizziness, insomnia, nausea.
- Severe side effects: Psychiatric symptoms, including hallucinations and delirium.
- Drug interactions: None of clinical significance.
- Warning: Not recommended for use in children under 1 year of age.
Alternative Drugs:
- Elderberry extract: Some studies suggest that elderberry extract may help reduce the duration and severity of influenza symptoms. Cost: $10-$20 for a 30-day supply.
- Vitamin C: High-dose vitamin C supplementation may have a modest effect on reducing the duration and severity of influenza symptoms. Cost: $5-$10 for a 30-day supply.
- Zinc: Zinc lozenges or syrup may help reduce the duration and severity of influenza symptoms. Cost: $5-$10 for a 30-day supply.
- Echinacea: Echinacea supplements may have immune-stimulating effects and could potentially reduce the risk of developing influenza. Cost: $10-$20 for a 30-day supply.
- Probiotics: Certain strains of probiotics may help support immune function and reduce the risk of respiratory infections. Cost: $10-$20 for a 30-day supply.
Surgical Procedures
- There are no surgical procedures indicated for the treatment of influenza.
Lifestyle Interventions
- Rest: Adequate rest is essential for the body to recover from influenza. Cost: None.
- Hydration: Drinking plenty of fluids helps prevent dehydration and supports the immune system. Cost: None.
- Steam inhalation: Inhaling steam can help relieve nasal congestion and soothe irritated airways. Cost: None.
- Warm saltwater gargles: Gargling with warm saltwater can help alleviate sore throat symptoms. Cost: None.
- Good nutrition: Consuming a balanced diet rich in fruits, vegetables, and lean proteins supports overall health and immune function. Cost: Varies depending on food choices.
It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
Mirari Cold Plasma Alternative Intervention
Understanding Mirari Cold Plasma
- Safe and Non-Invasive Treatment: Mirari Cold Plasma is a safe and non-invasive treatment option for various skin conditions. It does not require incisions, minimizing the risk of scarring, bleeding, or tissue damage.
- Efficient Extraction of Foreign Bodies: Mirari Cold Plasma facilitates the removal of foreign bodies from the skin by degrading and dissociating organic matter, allowing easier access and extraction.
- Pain Reduction and Comfort: Mirari Cold Plasma has a local analgesic effect, providing pain relief during the treatment, making it more comfortable for the patient.
- Reduced Risk of Infection: Mirari Cold Plasma has antimicrobial properties, effectively killing bacteria and reducing the risk of infection.
- Accelerated Healing and Minimal Scarring: Mirari Cold Plasma stimulates wound healing and tissue regeneration, reducing healing time and minimizing the formation of scars.
Mirari Cold Plasma Prescription
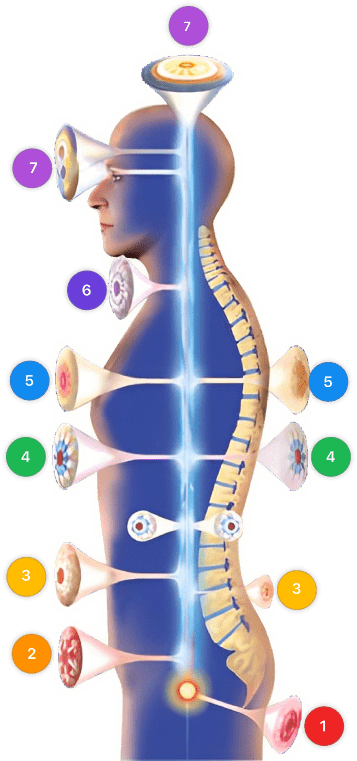
Video instructions for using Mirari Cold Plasma Device – R80 Influenza (ICD-10:J11.1)
| Mild | Moderate | Severe |
| Mode setting: 1 (Infection) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 1 (Infection) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 1 (Infection) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 3 (Antiviral Therapy) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 3 (Antiviral Therapy) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 3 (Antiviral Therapy) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Total Morning: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
| Usual treatment for 7-60 days approx. $140 USD – $1200 USD | Usual treatment for 6-8 weeks approx. $2,520 USD – $3,360 USD |
Usual treatment for 3-6 months approx. $5,400 USD – $10,800 USD
|
 |
|
Use the Mirari Cold Plasma device to treat Influenza effectively.
WARNING: MIRARI COLD PLASMA IS DESIGNED FOR THE HUMAN BODY WITHOUT ANY ARTIFICIAL OR THIRD PARTY PRODUCTS. USE OF OTHER PRODUCTS IN COMBINATION WITH MIRARI COLD PLASMA MAY CAUSE UNPREDICTABLE EFFECTS, HARM OR INJURY. PLEASE CONSULT A MEDICAL PROFESSIONAL BEFORE COMBINING ANY OTHER PRODUCTS WITH USE OF MIRARI.
Step 1: Cleanse the Skin
- Start by cleaning the affected area of the skin with a gentle cleanser or mild soap and water. Gently pat the area dry with a clean towel.
Step 2: Prepare the Mirari Cold Plasma device
- Ensure that the Mirari Cold Plasma device is fully charged or has fresh batteries as per the manufacturer’s instructions. Make sure the device is clean and in good working condition.
- Switch on the Mirari device using the power button or by following the specific instructions provided with the device.
- Some Mirari devices may have adjustable settings for intensity or treatment duration. Follow the manufacturer’s instructions to select the appropriate settings based on your needs and the recommended guidelines.
Step 3: Apply the Device
- Place the Mirari device in direct contact with the affected area of the skin. Gently glide or hold the device over the skin surface, ensuring even coverage of the area experiencing.
- Slowly move the Mirari device in a circular motion or follow a specific pattern as indicated in the user manual. This helps ensure thorough treatment coverage.
Step 4: Monitor and Assess:
- Keep track of your progress and evaluate the effectiveness of the Mirari device in managing your Influenza. If you have any concerns or notice any adverse reactions, consult with your health care professional.
Note
This guide is for informational purposes only and should not replace the advice of a medical professional. Always consult with your healthcare provider or a qualified medical professional for personal advice, diagnosis, or treatment. Do not solely rely on the information presented here for decisions about your health. Use of this information is at your own risk. The authors of this guide, nor any associated entities or platforms, are not responsible for any potential adverse effects or outcomes based on the content.
Mirari Cold Plasma System Disclaimer
- Purpose: The Mirari Cold Plasma System is a Class 2 medical device designed for use by trained healthcare professionals. It is registered for use in Thailand and Vietnam. It is not intended for use outside of these locations.
- Informational Use: The content and information provided with the device are for educational and informational purposes only. They are not a substitute for professional medical advice or care.
- Variable Outcomes: While the device is approved for specific uses, individual outcomes can differ. We do not assert or guarantee specific medical outcomes.
- Consultation: Prior to utilizing the device or making decisions based on its content, it is essential to consult with a Certified Mirari Tele-Therapist and your medical healthcare provider regarding specific protocols.
- Liability: By using this device, users are acknowledging and accepting all potential risks. Neither the manufacturer nor the distributor will be held accountable for any adverse reactions, injuries, or damages stemming from its use.
- Geographical Availability: This device has received approval for designated purposes by the Thai and Vietnam FDA. As of now, outside of Thailand and Vietnam, the Mirari Cold Plasma System is not available for purchase or use.
References
- Centers for Disease Control and Prevention. (2021). Influenza (Flu). Retrieved from https://www.cdc.gov/flu/index.htm
- World Health Organization. (2019). International Statistical Classification of Diseases and Related Health Problems (ICD-10).
- Paules, C., & Subbarao, K. (2017). Influenza. The Lancet, 390(10095), 697-708.
- Krammer, F., Smith, G. J. D., Fouchier, R. A. M., Peiris, M., Kedzierska, K., Doherty, P. C., … & García-Sastre, A. (2018). Influenza. Nature Reviews Disease Primers, 4(1), 1-21.
- Uyeki, T. M., Bernstein, H. H., Bradley, J. S., Englund, J. A., File, T. M., Fry, A. M., … & Pavia, A. T. (2019). Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clinical Infectious Diseases, 68(6), e1-e47.
- Treanor, J. J. (2016). Influenza (Including Avian Influenza and Swine Influenza). In Bennett, J. E., Dolin, R., & Blaser, M. J. (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (8th ed., pp. 2000-2024). Elsevier.
- Vemula, S. V., Zhao, J., Liu, J., Wang, X., Biswas, S., & Hewlett, I. (2016). Current Approaches for Diagnosis of Influenza Virus Infections in Humans. Viruses, 8(4), 96.
- Koo, H. J., Lim, S., Choe, J., Choi, S. H., Sung, H., & Do, K. H. (2018). Radiographic and CT Features of Viral Pneumonia. Radiographics, 38(3), 719-739.
- Brendish, N. J., Malachira, A. K., Armstrong, L., Houghton, R., Aitken, S., Nyimbili, E., … & Clark, T. W. (2017). Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. The Lancet Respiratory Medicine, 5(5), 401-411.
- Grohskopf, L. A., Alyanak, E., Broder, K. R., Blanton, L. H., Fry, A. M., Jernigan, D. B., & Atmar, R. L. (2020). Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2020-21 Influenza Season. MMWR Recommendations and Reports, 69(8), 1-24.
Related articles
Made in USA




























