
Introduction
Tobacco abuse is a significant public health issue that affects millions of individuals worldwide. It is characterized by the excessive use of tobacco products, such as cigarettes, cigars, and smokeless tobacco.[1] The aim of this guide is to provide healthcare professionals with a comprehensive overview of the diagnosis and management of tobacco abuse.
Codes
Symptoms
- Persistent cough
- Shortness of breath
- Chest pain
- Increased phlegm production
- Fatigue
- Decreased exercise tolerance
- Recurrent respiratory infections
- Hoarseness
- Bad breath
- Yellowing of teeth and nails[3]
Causes
- Nicotine addiction: Nicotine is a highly addictive substance found in tobacco products that leads to dependence and continued use.[4]
- Social and environmental factors: Peer pressure, exposure to tobacco use in the family or community, and easy access to tobacco products contribute to tobacco abuse.[5]
Diagnostic Steps
Medical History
- Ask about tobacco use history, including the type of tobacco products used, duration of use, and frequency of use.
- Inquire about previous quit attempts and the use of cessation aids.
- Assess the patient’s motivation to quit and readiness for change.
- Evaluate the patient’s knowledge about the health risks associated with tobacco use.[6]
Physical Examination
- Auscultate the lungs for abnormal breath sounds, such as wheezing or crackles.
- Assess the patient’s respiratory effort and breathing pattern.
- Inspect the oral cavity for signs of tobacco use, such as yellowing of teeth and oral lesions.
- Examine the skin for nicotine stains or discoloration.[7]
Laboratory Tests
- Cotinine levels: Cotinine is a metabolite of nicotine and can be measured in urine, blood, or saliva to confirm tobacco use.
- Pulmonary function tests: Spirometry can assess lung function and detect airflow limitation.
- Complete blood count: An elevated white blood cell count may indicate chronic inflammation associated with tobacco use.
- Carbon monoxide levels: Carbon monoxide is a byproduct of tobacco combustion and can be measured in exhaled breath to assess recent tobacco exposure.[8]
Diagnostic Imaging
- Chest X-ray: A chest X-ray can reveal signs of chronic obstructive pulmonary disease (COPD) or lung cancer.
- Computed tomography (CT) scan: A CT scan may be indicated if lung cancer is suspected or to evaluate the extent of lung damage in severe cases of tobacco abuse.[9]
Other Tests
- Nicotine dependence assessment: Various questionnaires, such as the Fagerström Test for Nicotine Dependence, can help assess the severity of nicotine addiction.
- Psychological assessment: Evaluate the patient’s mental health status, including the presence of anxiety, depression, or other psychiatric disorders that may contribute to tobacco abuse.[10]
Follow-up and Patient Education
- Schedule regular follow-up visits to monitor progress, provide support, and adjust treatment plans if necessary.
- Offer counseling and behavioral interventions to help patients quit tobacco use.
- Educate patients about the health risks associated with tobacco use and the benefits of quitting.
- Provide resources and referrals to smoking cessation programs, support groups, and helplines.
Possible Interventions
Traditional Interventions
Medications:
Top 5 drugs for Tobacco abuse:
- Nicotine replacement therapy (NRT):
- Cost: $30-$50 for a two-week supply.
- Contraindications: Severe cardiovascular disease, recent myocardial infarction, and severe arrhythmias.
- Side effects: Skin irritation, nausea, and headache.
- Severe side effects: Allergic reactions, palpitations, and chest pain.
- Drug interactions: None significant.
- Warning: Use caution in patients with underlying cardiovascular disease.
- Bupropion (Zyban):
- Cost: $100-$150 for a one-month supply.
- Contraindications: Seizure disorder, eating disorders, and current or previous use of monoamine oxidase inhibitors (MAOIs).
- Side effects: Dry mouth, insomnia, and headache.
- Severe side effects: Seizures, severe allergic reactions, and suicidal thoughts.
- Drug interactions: MAOIs, antipsychotics, and antidepressants.
- Warning: Monitor for neuropsychiatric symptoms and suicidality.
- Varenicline (Chantix):
- Cost: $200-$300 for a one-month supply.
- Contraindications: History of psychiatric illness, seizures, and pregnancy.
- Side effects: Nausea, insomnia, and abnormal dreams.
- Severe side effects: Suicidal thoughts, aggression, and mood changes.
- Drug interactions: Insulin, warfarin, and theophylline.
- Warning: Monitor for neuropsychiatric symptoms and suicidality.
- Clonidine:
- Cost: $20-$40 for a one-month supply.
- Contraindications: Hypotension, bradycardia, and heart block.
- Side effects: Dry mouth, drowsiness, and constipation.
- Severe side effects: Hypotension, bradycardia, and rebound hypertension.
- Drug interactions: Beta-blockers, calcium channel blockers, and opioids.
- Warning: Monitor blood pressure and heart rate.
- Nortriptyline:
- Cost: $20-$40 for a one-month supply.
- Contraindications: Recent myocardial infarction, heart block, and glaucoma.
- Side effects: Dry mouth, constipation, and sedation.
- Severe side effects: Cardiac arrhythmias, seizures, and serotonin syndrome.
- Drug interactions: MAOIs, SSRIs, and antipsychotics.
- Warning: Monitor for cardiac arrhythmias and suicidality.
Behavioral Interventions:
- Cognitive-behavioral therapy (CBT): Helps patients identify and modify thoughts, behaviors, and triggers associated with tobacco use.
- Motivational interviewing: A patient-centered approach that aims to enhance motivation and commitment to quitting tobacco use.
- Support groups: Provide a supportive environment for individuals to share experiences, receive encouragement, and learn coping strategies.
Alternative Interventions
- Acupuncture: May help reduce tobacco cravings and withdrawal symptoms. Cost: $60-$120 per session.
- Hypnotherapy: Uses hypnosis to modify thoughts and behaviors related to tobacco use. Cost: $75-$150 per session.
- Mindfulness-based interventions: Teach individuals to be aware of cravings and develop strategies to manage them. Cost: Varies depending on the program.
- Herbal supplements: Some herbs, such as lobelia and St. John’s wort, are believed to reduce tobacco cravings. Cost: Varies depending on the specific supplement.
Lifestyle Interventions
- Exercise: Regular physical activity can help reduce tobacco cravings and improve overall well-being. Cost: Varies depending on the chosen activity.
- Healthy diet: A balanced diet rich in fruits, vegetables, and whole grains may help reduce tobacco cravings. Cost: Varies depending on food choices.
- Stress management techniques: Practices such as meditation, deep breathing exercises, and yoga can help individuals cope with stress without turning to tobacco. Cost: Varies depending on the chosen method.
- Support from family and friends: Having a strong support system can greatly increase the chances of successfully quitting tobacco use. Cost: None.
It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
Mirari Cold Plasma Alternative Intervention
Understanding Mirari Cold Plasma
- Safe and Non-Invasive Treatment: Mirari Cold Plasma is a safe and non-invasive treatment option for various skin conditions. It does not require incisions, minimizing the risk of scarring, bleeding, or tissue damage.
- Efficient Extraction of Foreign Bodies: Mirari Cold Plasma facilitates the removal of foreign bodies from the skin by degrading and dissociating organic matter, allowing easier access and extraction.
- Pain Reduction and Comfort: Mirari Cold Plasma has a local analgesic effect, providing pain relief during the treatment, making it more comfortable for the patient.
- Reduced Risk of Infection: Mirari Cold Plasma has antimicrobial properties, effectively killing bacteria and reducing the risk of infection.
- Accelerated Healing and Minimal Scarring: Mirari Cold Plasma stimulates wound healing and tissue regeneration, reducing healing time and minimizing the formation of scars.
Mirari Cold Plasma Prescription
Video instructions for using Mirari Cold Plasma Device – P17 Tobacco abuse (ICD-10:F17.2)
| Mild | Moderate | Severe |
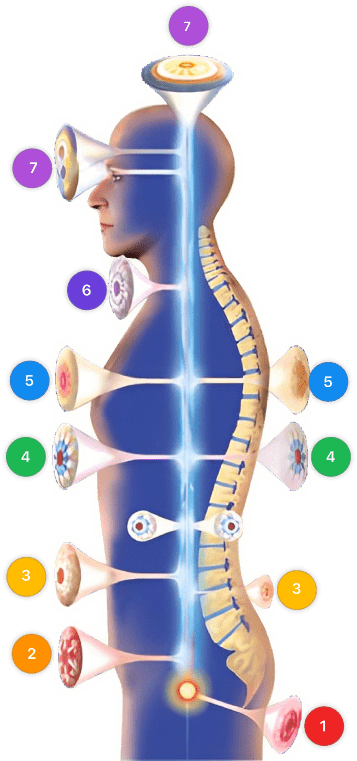
| Mode setting: 2 (Wound Healing) Location: 5 (Lungs) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 2 (Wound Healing) Location: 5 (Lungs) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 2 (Wound Healing) Location: 5 (Lungs) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 2 (Wound Healing) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Total Morning: 30 minutes approx. $5 USD, Evening: 30 minutes approx. $5 USD |
Total Morning: 60 minutes approx. $10 USD, Lunch: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD, |
Total Morning: 60 minutes approx. $10 USD, Lunch: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD, |
| Usual treatment for 7-60 days approx. $70 USD – $600 USD | Usual treatment for 6-8 weeks approx. $1,260 USD – $1,680 USD |
Usual treatment for 3-6 months approx. $2,700 USD – $5,400 USD
|
 |
|
Use the Mirari Cold Plasma device to treat Tobacco abuse effectively.
WARNING: MIRARI COLD PLASMA IS DESIGNED FOR THE HUMAN BODY WITHOUT ANY ARTIFICIAL OR THIRD PARTY PRODUCTS. USE OF OTHER PRODUCTS IN COMBINATION WITH MIRARI COLD PLASMA MAY CAUSE UNPREDICTABLE EFFECTS, HARM OR INJURY. PLEASE CONSULT A MEDICAL PROFESSIONAL BEFORE COMBINING ANY OTHER PRODUCTS WITH USE OF MIRARI.
Step 1: Cleanse the Skin
- Start by cleaning the affected area of the skin with a gentle cleanser or mild soap and water. Gently pat the area dry with a clean towel.
Step 2: Prepare the Mirari Cold Plasma device
- Ensure that the Mirari Cold Plasma device is fully charged or has fresh batteries as per the manufacturer’s instructions. Make sure the device is clean and in good working condition.
- Switch on the Mirari device using the power button or by following the specific instructions provided with the device.
- Some Mirari devices may have adjustable settings for intensity or treatment duration. Follow the manufacturer’s instructions to select the appropriate settings based on your needs and the recommended guidelines.
Step 3: Apply the Device
- Place the Mirari device in direct contact with the affected area of the skin. Gently glide or hold the device over the skin surface, ensuring even coverage of the area experiencing.
- Slowly move the Mirari device in a circular motion or follow a specific pattern as indicated in the user manual. This helps ensure thorough treatment coverage.
Step 4: Monitor and Assess:
- Keep track of your progress and evaluate the effectiveness of the Mirari device in managing your Tobacco abuse. If you have any concerns or notice any adverse reactions, consult with your health care professional.
Note
This guide is for informational purposes only and should not replace the advice of a medical professional. Always consult with your healthcare provider or a qualified medical professional for personal advice, diagnosis, or treatment. Do not solely rely on the information presented here for decisions about your health. Use of this information is at your own risk. The authors of this guide, nor any associated entities or platforms, are not responsible for any potential adverse effects or outcomes based on the content.
Mirari Cold Plasma System Disclaimer
- Purpose: The Mirari Cold Plasma System is a Class 2 medical device designed for use by trained healthcare professionals. It is registered for use in Thailand and Vietnam. It is not intended for use outside of these locations.
- Informational Use: The content and information provided with the device are for educational and informational purposes only. They are not a substitute for professional medical advice or care.
- Variable Outcomes: While the device is approved for specific uses, individual outcomes can differ. We do not assert or guarantee specific medical outcomes.
- Consultation: Prior to utilizing the device or making decisions based on its content, it is essential to consult with a Certified Mirari Tele-Therapist and your medical healthcare provider regarding specific protocols.
- Liability: By using this device, users are acknowledging and accepting all potential risks. Neither the manufacturer nor the distributor will be held accountable for any adverse reactions, injuries, or damages stemming from its use.
- Geographical Availability: This device has received approval for designated purposes by the Thai and Vietnam FDA. As of now, outside of Thailand and Vietnam, the Mirari Cold Plasma System is not available for purchase or use.
References
- World Health Organization. (2021). Tobacco. Retrieved from https://www.who.int/news-room/fact-sheets/detail/tobacco
- World Health Organization. (2019). International Statistical Classification of Diseases and Related Health Problems (ICD-10).
- U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General.
- Benowitz, N. L. (2010). Nicotine addiction. New England Journal of Medicine, 362(24), 2295-2303.
- U.S. Department of Health and Human Services. (2012). Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General.
- Fiore, M. C., Jaén, C. R., Baker, T. B., et al. (2008). Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline.
- Tomar, S. L., Asma, S., & Westphal, L. (2009). Effects of tobacco use on oral health. American Journal of the Medical Sciences, 338(4), 255-259.
- Benowitz, N. L., Hukkanen, J., & Jacob, P. (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology, (192), 29-60.
- National Lung Screening Trial Research Team. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine, 365(5), 395-409.
- Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerström, K. O. (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119-1127.
Related articles
Made in USA




























