
Introduction
COVID-19 is a highly contagious respiratory illness caused by the novel coronavirus SARS-CoV-2. It has become a global pandemic, leading to significant morbidity and mortality worldwide.[1] The aim of this guide is to provide healthcare professionals with a comprehensive overview of the symptoms, causes, diagnostic steps, possible interventions, and patient education related to COVID-19.
Codes
Symptoms
- Fever: Persistent high body temperature (above 100.4°F or 38°C).
- Cough: Dry or productive cough that may worsen over time.
- Shortness of breath: Difficulty breathing or rapid breathing.
- Fatigue: Feeling tired or exhausted.
- Muscle or body aches: Generalized pain or discomfort.
- Headache: Pain or pressure in the head.
- Sore throat: Pain or irritation in the throat.
- Loss of taste or smell: Inability to taste or smell.
- Congestion or runny nose: Nasal congestion or excessive nasal discharge.
- Nausea or vomiting: Feeling sick to the stomach or vomiting.
- Diarrhea: Loose or watery stools.[4]
Causes
- SARS-CoV-2 infection: COVID-19 is primarily caused by the transmission of the SARS-CoV-2 virus through respiratory droplets from an infected person. It can also spread by touching contaminated surfaces and then touching the face.[5]
Diagnostic Steps
Medical History
- Gather information about the patient’s recent travel history, exposure to individuals with confirmed COVID-19, and any symptoms they may be experiencing.
- Identify any underlying medical conditions or risk factors that may increase the severity of COVID-19 symptoms.[6]
Physical Examination
- Conduct a thorough physical examination, paying close attention to respiratory symptoms such as cough, shortness of breath, and auscultation of lung sounds.
- Check for fever, elevated heart rate, and signs of dehydration.[7]
Laboratory Tests
- Nasopharyngeal swab for RT-PCR testing: This is the gold standard diagnostic test for COVID-19, detecting the presence of viral RNA.
- Rapid antigen test: A quick test that detects specific viral proteins to determine if a person is currently infected with SARS-CoV-2.
- Antibody testing: Detects the presence of antibodies against SARS-CoV-2, indicating past infection or immune response.[8]
Diagnostic Imaging
- Chest X-ray: May show signs of pneumonia or lung inflammation.
- CT scan of the chest: Can provide a more detailed evaluation of lung involvement and severity of disease.[9]
Other Tests
- Blood tests: Complete blood count (CBC), C-reactive protein (CRP), and D-dimer levels can help assess the severity of inflammation and coagulation abnormalities.
- Pulse oximetry: Measure oxygen saturation levels to assess respiratory function.
- Electrocardiogram (ECG): Evaluate cardiac function and detect any abnormalities, such as arrhythmias or myocardial injury.[10]
Follow-up and Patient Education
- Provide clear instructions on self-isolation, quarantine, and when to seek medical attention.
- Educate patients on proper hand hygiene, respiratory etiquette, and the importance of wearing masks in public settings.
- Discuss the potential complications of COVID-19 and the importance of monitoring symptoms.[1][4]
Possible Interventions
Traditional Interventions
Medications:
Top 5 drugs for COVID-19:
- Monoclonal Antibodies (e.g., bamlanivimab, casirivimab, and imdevimab) :
- Cost Range: Varies, but in the US, can exceed $1,000 for a dose.
- Contraindications: Known hypersensitivity to the active substance or any of the excipients.
- Side Effects: Nausea, diarrhea, dizziness.
- Severe Side Effects: Anaphylaxis and infusion-related reactions.
- Drug Interactions: Not known to interact with other drugs, but more studies might be necessary.
- Warning: Should be administered under healthcare professional supervision due to potential for serious reactions.
- Ultraviolet (UV) Light Therapy
- Cost: Not standardized; varies by technology and region.
- Contraindications: Potential tissue damage from direct UV exposure.
- Side Effects: Burns, skin irritation, skin discoloration, discomfort.
- Severe Side Effects: Risk of skin cancers, eye damage, internal tissue damage.
- Drug Interactions: Some drugs may cause photosensitivity, heightening UV damage risk.
- Warning: UV light can harm human tissues. Any proposed UV treatment should be approached with caution and in consultation with medical professionals.
- Dexamethasone:
- Cost: $0.10 per tablet.
- Contraindications: Systemic fungal infections.
- Side effects: Increased appetite, weight gain, mood changes.
- Severe side effects: Adrenal insufficiency, immunosuppression.
- Drug interactions: Strong CYP3A4 inducers.
- Warning: Use with caution in patients with diabetes or psychiatric disorders.
- Convalescent plasma:
- Cost: Varies depending on the blood bank and location.
- Contraindications: Severe allergic reactions to plasma products.
- Side effects: Allergic reactions, transfusion-related acute lung injury.
- Severe side effects: Transfusion-associated circulatory overload, transfusion-related acute lung injury.
- Drug interactions: None.
- Warning: Monitor for signs of transfusion reactions during administration.
- Tocilizumab:
- Cost: Prices for human use can vary widely based on the country and healthcare setting but typically range from $10 to $100 for a course of treatment.
- Contraindications: Known hypersensitivity to albendazole or similar drugs. It should also be used with caution in pregnant women due to potential teratogenic effects.
- Side Effects: Abdominal pain, nausea, headache, dizziness, elevated liver enzymes.
- Severe Side Effects: Rarely, bone marrow suppression, severe skin reactions, and liver problems.
- Drug Interactions: Cimetidine, praziquantel, and dexamethasone may increase levels of albendazole. Antiepileptics like carbamazepine, phenytoin, and phenobarbital may decrease its levels.
- Warning: Always use under medical supervision. It’s not intended or approved for Marburg virus treatment.
Alternative Drugs:
- Hydroxychloroquine: Controversial drug with limited evidence of efficacy.
- Ivermectin: Antiparasitic drug with potential antiviral properties.
- Favipiravir: Broad-spectrum antiviral medication.
- Azithromycin: Antibiotic with potential antiviral effects.
- Lopinavir/Ritonavir: Antiretroviral combination used in HIV treatment.
Surgical Procedures:
- There are currently no surgical procedures specifically indicated for the treatment of COVID-19.
Alternative Interventions
- Vitamin C supplementation: May help boost the immune system. Cost: $10-$20 per month.
- Zinc supplementation: May have antiviral properties. Cost: $5-$10 per month.
- Herbal remedies (e.g., elderberry, echinacea): Some herbs may have immune-boosting properties. Cost: Varies depending on the specific supplement.
- Steam inhalation: May help relieve respiratory symptoms. Cost: Minimal.
- Probiotics: May support immune function. Cost: $10-$30 per month.
Lifestyle Interventions
- Adequate rest and sleep: Essential for immune system function. Cost: None.
- Regular exercise: Helps improve overall health and immune function. Cost: None or minimal (gym membership, equipment).
- Healthy diet: Provides essential nutrients for immune support. Cost: Varies depending on food choices.
- Stress management techniques (e.g., meditation, yoga): Can help reduce stress and boost immune function. Cost: Minimal or none.
- Avoidance of tobacco and alcohol: Both can weaken the immune system. Cost: None. It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
Mirari Cold Plasma Alternative Intervention
Understanding Mirari Cold Plasma
- Safe and Non-Invasive Treatment: Mirari Cold Plasma is a safe and non-invasive treatment option for various skin conditions. It does not require incisions, minimizing the risk of scarring, bleeding, or tissue damage.
- Efficient Extraction of Foreign Bodies: Mirari Cold Plasma facilitates the removal of foreign bodies from the skin by degrading and dissociating organic matter, allowing easier access and extraction.
- Pain Reduction and Comfort: Mirari Cold Plasma has a local analgesic effect, providing pain relief during the treatment, making it more comfortable for the patient.
- Reduced Risk of Infection: Mirari Cold Plasma has antimicrobial properties, effectively killing bacteria and reducing the risk of infection.
- Accelerated Healing and Minimal Scarring: Mirari Cold Plasma stimulates wound healing and tissue regeneration, reducing healing time and minimizing the formation of scars.
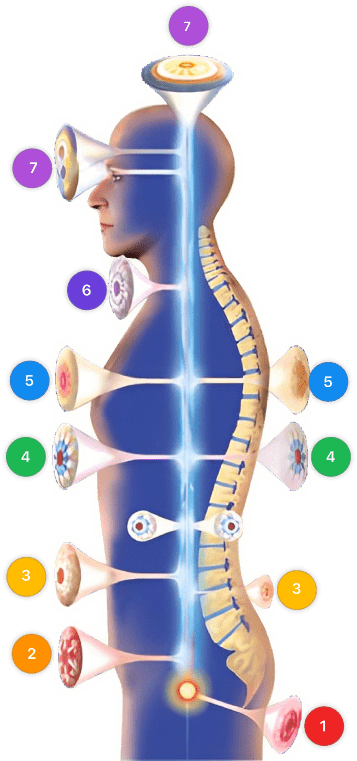
Mirari Cold Plasma Prescription
| Mild | Moderate | Severe |
| Mode setting: 1 (Infection) Location: Localized Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 1 (Infection) Location: Localized Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 1 (Infection) Location: Localized Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 2 (Wound) Location: 7 (Neuro system & ENT) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 2 (Wound) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 2 (Wound) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 3 (Antiviral) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 3 (Antiviral) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 3 (Antiviral) Location: 6 (Throat, Lymphatic & Thyroid) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immuno) Location: 1 (Sacrum) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immuno) Location: 1 (Sacrum) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immuno) Location: 1 (Sacrum) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Total Morning: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
| Usual treatment for 7-60 days approx. $140 USD – $1200 USD | Usual treatment for 6-8 weeks approx. $2,520 USD – $3,360 USD |
Usual treatment for 3-6 months approx. $5,400 USD – $10,800 USD
|
 |
|
Use the Mirari Cold Plasma device to treat COVID-19 effectively.
WARNING: MIRARI COLD PLASMA IS DESIGNED FOR THE HUMAN BODY WITHOUT ANY ARTIFICIAL OR THIRD PARTY PRODUCTS. USE OF OTHER PRODUCTS IN COMBINATION WITH MIRARI COLD PLASMA MAY CAUSE UNPREDICTABLE EFFECTS, HARM OR INJURY. PLEASE CONSULT A MEDICAL PROFESSIONAL BEFORE COMBINING ANY OTHER PRODUCTS WITH USE OF MIRARI.
Step 1: Cleanse the Skin
- Start by cleaning the affected area of the skin with a gentle cleanser or mild soap and water. Gently pat the area dry with a clean towel.
Step 2: Prepare the Mirari Cold Plasma device
- Ensure that the Mirari Cold Plasma device is fully charged or has fresh batteries as per the manufacturer’s instructions. Make sure the device is clean and in good working condition.
- Switch on the Mirari device using the power button or by following the specific instructions provided with the device.
- Some Mirari devices may have adjustable settings for intensity or treatment duration. Follow the manufacturer’s instructions to select the appropriate settings based on your needs and the recommended guidelines.
Step 3: Apply the Device
- Place the Mirari device in direct contact with the affected area of the skin. Gently glide or hold the device over the skin surface, ensuring even coverage of the area experiencing.
- Slowly move the Mirari device in a circular motion or follow a specific pattern as indicated in the user manual. This helps ensure thorough treatment coverage.
Step 4: Monitor and Assess:
- Keep track of your progress and evaluate the effectiveness of the Mirari device in managing your COVID-19. If you have any concerns or notice any adverse reactions, consult with your health care professional.
Note
This guide is for informational purposes only and should not replace the advice of a medical professional. Always consult with your healthcare provider or a qualified medical professional for personal advice, diagnosis, or treatment. Do not solely rely on the information presented here for decisions about your health. Use of this information is at your own risk. The authors of this guide, nor any associated entities or platforms, are not responsible for any potential adverse effects or outcomes based on the content.
Mirari Cold Plasma System Disclaimer
- Purpose: The Mirari Cold Plasma System is a Class 2 medical device designed for use by trained healthcare professionals. It is registered for use in Thailand and Vietnam. It is not intended for use outside of these locations.
- Informational Use: The content and information provided with the device are for educational and informational purposes only. They are not a substitute for professional medical advice or care.
- Variable Outcomes: While the device is approved for specific uses, individual outcomes can differ. We do not assert or guarantee specific medical outcomes.
- Consultation: Prior to utilizing the device or making decisions based on its content, it is essential to consult with a Certified Mirari Tele-Therapist and your medical healthcare provider regarding specific protocols.
- Liability: By using this device, users are acknowledging and accepting all potential risks. Neither the manufacturer nor the distributor will be held accountable for any adverse reactions, injuries, or damages stemming from its use.
- Geographical Availability: This device has received approval for designated purposes by the Thai and Vietnam FDA. As of now, outside of Thailand and Vietnam, the Mirari Cold Plasma System is not available for purchase or use.
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- World Health Organization. International Classification of Primary Care, Second edition (ICPC-2). Geneva: WHO; 2003.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Geneva: WHO; 2019.
- Centers for Disease Control and Prevention. Symptoms of COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- Centers for Disease Control and Prevention. How COVID-19 Spreads. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- National Institutes of Health. Clinical Spectrum of SARS-CoV-2 Infection. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- Infectious Diseases Society of America. Guidelines on the Diagnosis of COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/
- Food and Drug Administration. Coronavirus Testing Basics. Available at: https://www.fda.gov/consumers/consumer-updates/coronavirus-testing-basics
- Radiological Society of North America. RSNA COVID-19 Resources. Available at: https://www.rsna.org/covid-19
- American College of Cardiology. COVID-19 Clinical Guidance For the Cardiovascular Care Team. Available at: https://www.acc.org/latest-in-cardiology/features/accs-coronavirus-disease-2019-covid-19-hub
Related articles
Made in USA



























