
Introduction
Viral hepatitis is a condition characterized by inflammation of the liver caused by a viral infection. It is a significant global health issue, with millions of people affected worldwide[1]. The aim of this guide is to provide healthcare professionals with a comprehensive overview of the diagnosis and management of viral hepatitis.
Codes
Symptoms
- Fatigue: Patients may experience persistent tiredness and lack of energy[2].
- Jaundice: Yellowing of the skin and eyes due to liver dysfunction[3].
- Abdominal pain: Discomfort or pain in the upper right quadrant of the abdomen[4].
- Nausea and vomiting: Some patients may experience gastrointestinal symptoms[5].
- Loss of appetite: A decrease in appetite and weight loss may occur[6].
- Dark urine: Urine may appear dark or tea-colored[7].
- Pale stools: Stools may become pale or clay-colored[8].
Causes
- Hepatitis A virus (HAV): Transmitted through contaminated food or water[9].
- Hepatitis B virus (HBV): Transmitted through blood, sexual contact, or from mother to child during childbirth[10].
- Hepatitis C virus (HCV): Transmitted through blood, primarily through sharing needles or other drug paraphernalia.
- Hepatitis D virus (HDV): Can only infect individuals already infected with HBV.
- Hepatitis E virus (HEV): Transmitted through contaminated food or water, primarily in developing countries.
Diagnostic Steps
Medical History
- Gather information on risk factors such as intravenous drug use, unprotected sex, or travel to endemic areas.
- Inquire about symptoms such as fatigue, jaundice, abdominal pain, and changes in appetite.
- Assess for any underlying medical conditions or medications that may contribute to liver dysfunction.
Physical Examination
- Observe for signs of jaundice, such as yellowing of the skin and eyes.
- Palpate the abdomen for tenderness or enlargement of the liver.
- Check for signs of fluid accumulation, such as ascites or peripheral edema.
Laboratory Tests
- Hepatitis panel: Tests for the presence of antibodies and antigens specific to different hepatitis viruses.
- Liver function tests: Measure levels of liver enzymes, bilirubin, and albumin.
- Viral load testing: Determines the amount of viral genetic material in the blood.
- Serology testing: Detects specific antibodies against hepatitis viruses.
- Coagulation studies: Assess the liver’s ability to produce clotting factors.
Diagnostic Imaging
- Abdominal ultrasound: Evaluates the liver for signs of inflammation or scarring.
- CT scan or MRI: Provides detailed images of the liver and surrounding structures.
- FibroScan: Measures liver stiffness to assess the degree of fibrosis or cirrhosis.
Other Tests
- Liver biopsy: Invasive procedure to obtain a tissue sample for further evaluation.
- Polymerase chain reaction (PCR): Detects viral genetic material in the blood.
- Elastography: Non-invasive method to assess liver stiffness.
Follow-up and Patient Education
- Schedule regular follow-up appointments to monitor liver function and viral load.
- Educate patients on the importance of lifestyle modifications, such as avoiding alcohol and practicing safe sex.
- Provide information on support groups and resources for patients with viral hepatitis.
Possible Interventions
Traditional Interventions
Medications:
Top 5 drugs for Viral hepatitis:
- Interferon-alpha:
- Cost: $500-$1,000 per month.
- Contraindications: Autoimmune disorders, severe depression.
- Side effects: Flu-like symptoms, fatigue, depression.
- Severe side effects: Bone marrow suppression, liver failure.
- Drug interactions: None reported.
- Warning: Regular monitoring of blood counts and liver function required.
- Ribavirin:
- Cost: $500-$1,000 per month.
- Contraindications: Pregnancy, severe anemia.
- Side effects: Fatigue, anemia, rash.
- Severe side effects: Hemolytic anemia, birth defects.
- Drug interactions: None reported.
- Warning: Pregnancy must be avoided during treatment and for several months after.
- Direct-acting antivirals (DAAs) (e.g., Sofosbuvir, Ledipasvir, Daclatasvir):
- Cost: $20,000-$100,000 for a 12-week course.
- Contraindications: None reported.
- Side effects: Headache, fatigue, nausea.
- Severe side effects: None reported.
- Drug interactions: Varies depending on the specific DAA.
- Warning: Regular monitoring of liver function required during treatment.
- Entecavir:
- Cost: $500-$1,000 per month.
- Contraindications: None reported.
- Side effects: Headache, fatigue, dizziness.
- Severe side effects: Lactic acidosis, severe hepatomegaly.
- Drug interactions: None reported.
- Warning: Regular monitoring of liver function required.
- Tenofovir:
- Cost: $500-$1,000 per month.
- Contraindications: None reported.
- Side effects: Nausea, diarrhea, headache.
- Severe side effects: Lactic acidosis, severe hepatomegaly.
- Drug interactions: None reported.
- Warning: Regular monitoring of kidney function required.
Alternative Drugs:
- Silymarin (milk thistle extract): Thought to have hepatoprotective properties. Cost: $10-$30 per month.
- Lactoferrin: A natural protein with antiviral and immunomodulatory effects. Cost: $20-$50 per month.
- Vitamin E: Antioxidant that may help reduce liver inflammation. Cost: $5-$15 per month.
- Phyllanthus niruri: Herbal supplement with potential antiviral properties. Cost: $10-$30 per month.
- Licorice root: May have antiviral and anti-inflammatory effects. Cost: $10-$30 per month.
Surgical Procedures:
- Liver transplantation: Considered for patients with end-stage liver disease or hepatocellular carcinoma. Cost: $500,000-$800,000.
Alternative Interventions
- Acupuncture: May help alleviate symptoms and improve overall well-being. Cost: $60-$120 per session.
- Herbal supplements: Certain herbs, such as milk thistle and licorice root, may have hepatoprotective properties. Cost: Varies depending on the specific supplement.
- Yoga and meditation: Can help reduce stress and improve overall health. Cost: $10-$20 per class.
- Dietary modifications: A healthy diet low in fat and alcohol can support liver health. Cost: Varies depending on individual food choices.
- Exercise: Regular physical activity can improve liver function and overall well-being. Cost: Varies depending on individual preferences.
Lifestyle Interventions
- Avoid alcohol: Alcohol can worsen liver damage and interfere with treatment. Cost: Varies depending on individual preferences.
- Practice safe sex: Use barrier methods to prevent transmission of hepatitis B and C. Cost: Varies depending on individual preferences.
- Maintain a healthy weight: Obesity can contribute to liver disease progression. Cost: Varies depending on individual preferences.
- Get vaccinated: Vaccines are available for hepatitis A and B. Cost: Varies depending on healthcare coverage.
- Avoid sharing needles: Intravenous drug use is a major risk factor for hepatitis transmission. Cost: Varies depending on individual preferences.
It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
Mirari Cold Plasma Alternative Intervention
Understanding Mirari Cold Plasma
- Safe and Non-Invasive Treatment: Mirari Cold Plasma is a safe and non-invasive treatment option for various skin conditions. It does not require incisions, minimizing the risk of scarring, bleeding, or tissue damage.
- Efficient Extraction of Foreign Bodies: Mirari Cold Plasma facilitates the removal of foreign bodies from the skin by degrading and dissociating organic matter, allowing easier access and extraction.
- Pain Reduction and Comfort: Mirari Cold Plasma has a local analgesic effect, providing pain relief during the treatment, making it more comfortable for the patient.
- Reduced Risk of Infection: Mirari Cold Plasma has antimicrobial properties, effectively killing bacteria and reducing the risk of infection.
- Accelerated Healing and Minimal Scarring: Mirari Cold Plasma stimulates wound healing and tissue regeneration, reducing healing time and minimizing the formation of scars.
Mirari Cold Plasma Prescription
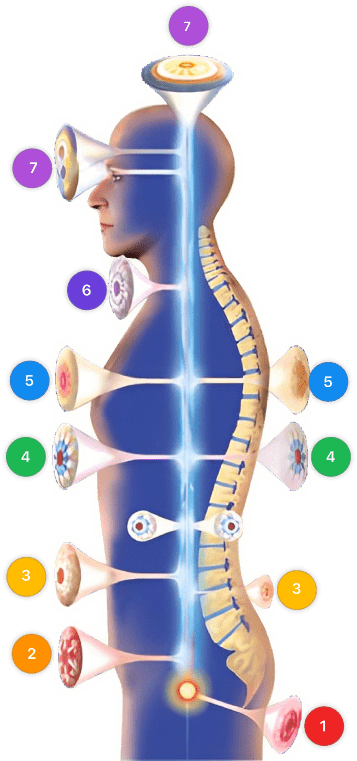
Video instructions for using Mirari Cold Plasma Device – D72 Viral hepatitis (ICD-10:B15, B16, B17, B18, B19)
| Mild | Moderate | Severe |
| Mode setting: 1 (Infection) Location: 3 (Kidney, Liver & Spleen) Morning: 15 minutes, Evening: 15 minutes | Mode setting: 1 (Infection) Location: 3 (Kidney, Liver & Spleen) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes | Mode setting: 1 (Infection) Location: 3 (Kidney, Liver & Spleen) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 3 (Antiviral Therapy) Location: 3 (Kidney, Liver & Spleen) Morning: 15 minutes, Evening: 15 minutes | Mode setting: 3 (Antiviral Therapy) Location: 3 (Kidney, Liver & Spleen) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes | Mode setting: 3 (Antiviral Therapy) Location: 3 (Kidney, Liver & Spleen) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 3 (Antiviral Therapy) Location: 0 (Localized) Morning: 15 minutes, Evening: 15 minutes | Mode setting: 3 (Antiviral Therapy) Location: 0 (Localized) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes | Mode setting: 3 (Antiviral Therapy) Location: 0 (Localized) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 15 minutes, Evening: 15 minutes | Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes | Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Total Morning: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD | Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, | Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
| Usual treatment for 7-60 days approx. $140 USD – $1200 USD | Usual treatment for 6-8 weeks approx. $2,520USD – $3,360 USD | Usual treatment for 3-6 months approx. $5,400 USD – $10,800 USD |
 |
|
Use the Mirari Cold Plasma device to treat Viral hepatitis effectively.
WARNING: MIRARI COLD PLASMA IS DESIGNED FOR THE HUMAN BODY WITHOUT ANY ARTIFICIAL OR THIRD PARTY PRODUCTS. USE OF OTHER PRODUCTS IN COMBINATION WITH MIRARI COLD PLASMA MAY CAUSE UNPREDICTABLE EFFECTS, HARM OR INJURY. PLEASE CONSULT A MEDICAL PROFESSIONAL BEFORE COMBINING ANY OTHER PRODUCTS WITH USE OF MIRARI.
Step 1: Cleanse the Skin
- Start by cleaning the affected area of the skin with a gentle cleanser or mild soap and water. Gently pat the area dry with a clean towel.
Step 2: Prepare the Mirari Cold Plasma device
- Ensure that the Mirari Cold Plasma device is fully charged or has fresh batteries as per the manufacturer’s instructions. Make sure the device is clean and in good working condition.
- Switch on the Mirari device using the power button or by following the specific instructions provided with the device.
- Some Mirari devices may have adjustable settings for intensity or treatment duration. Follow the manufacturer’s instructions to select the appropriate settings based on your needs and the recommended guidelines.
Step 3: Apply the Device
- Place the Mirari device in direct contact with the affected area of the skin. Gently glide or hold the device over the skin surface, ensuring even coverage of the area experiencing.
- Slowly move the Mirari device in a circular motion or follow a specific pattern as indicated in the user manual. This helps ensure thorough treatment coverage.
Step 4: Monitor and Assess:
- Keep track of your progress and evaluate the effectiveness of the Mirari device in managing your Viral hepatitis. If you have any concerns or notice any adverse reactions, consult with your health care professional.
Note
This guide is for informational purposes only and should not replace the advice of a medical professional. Always consult with your healthcare provider or a qualified medical professional for personal advice, diagnosis, or treatment. Do not solely rely on the information presented here for decisions about your health. Use of this information is at your own risk. The authors of this guide, nor any associated entities or platforms, are not responsible for any potential adverse effects or outcomes based on the content.
Mirari Cold Plasma System Disclaimer
- Purpose: The Mirari Cold Plasma System is a Class 2 medical device designed for use by trained healthcare professionals. It is registered for use in Thailand and Vietnam. It is not intended for use outside of these locations.
- Informational Use: The content and information provided with the device are for educational and informational purposes only. They are not a substitute for professional medical advice or care.
- Variable Outcomes: While the device is approved for specific uses, individual outcomes can differ. We do not assert or guarantee specific medical outcomes.
- Consultation: Prior to utilizing the device or making decisions based on its content, it is essential to consult with a Certified Mirari Tele-Therapist and your medical healthcare provider regarding specific protocols.
- Liability: By using this device, users are acknowledging and accepting all potential risks. Neither the manufacturer nor the distributor will be held accountable for any adverse reactions, injuries, or damages stemming from its use.
- Geographical Availability: This device has received approval for designated purposes by the Thai and Vietnam FDA. As of now, outside of Thailand and Vietnam, the Mirari Cold Plasma System is not available for purchase or use.
References
- World Health Organization. (2021). Hepatitis.//www.who.int/health-topics/hepatitis
- Terrault, N. A., Lok, A. S., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., … & Wong, J. B. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology, 67(4), 1560-1599.
- European Association for the Study of the Liver. (2017). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology, 67(2), 370-398.
- Ghany, M. G., Morgan, T. R., & AASLD‐IDSA Hepatitis C Guidance Panel. (2020). Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology, 71(2), 686-721.
- Kamar, N., Izopet, J., Pavio, N., Aggarwal, R., Labrique, A., Wedemeyer, H., & Dalton, H. R. (2017). Hepatitis E virus infection. Nature reviews Disease primers, 3(1), 1-16.
- Wedemeyer, H., Pischke, S., & Manns, M. P. (2012). Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology, 142(6), 1388-1397.
- Rizzetto, M. (2015). Hepatitis D virus: introduction and epidemiology. Cold Spring Harbor perspectives in medicine, 5(7), a021576.
- Feld, J. J., Jacobson, I. M., Hézode, C., Asselah, T., Ruane, P. J., Gruener, N., … & ASTRAL-1 Investigators. (2015). Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. New England Journal of Medicine, 373(27), 2599-2607.
- Jacobson, I. M., Gordon, S. C., Kowdley, K. V., Yoshida, E. M., Rodriguez-Torres, M., Sulkowski, M. S., … & POSITRON Study. (2013). Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New England Journal of Medicine, 368(20), 1867-1877.
- Afdhal, N., Zeuzem, S., Kwo, P., Chojkier, M., Gitlin, N., Puoti, M., … & ION-1 Investigators. (2014). Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New England Journal of Medicine, 370(20), 1889-1898.
Related articles
Made in USA




























