
Introduction
Fear of cancer in the neurological system is a common concern among patients. This guide aims to provide a comprehensive overview of the diagnostic steps and possible interventions for this condition.
Codes
- ICPC-2 Code: N26 Fear cancer neurological system[1]
- ICD-10 Code: Z71.1 Person with feared complaint in whom no diagnosis is made[2]
Symptoms
- Persistent headaches: Patients may experience frequent or severe headaches that do not respond to over-the-counter pain medications.
- Changes in vision: Blurred vision, double vision, or loss of vision may occur.
- Difficulty with balance and coordination: Patients may have trouble walking or maintaining balance.
- Seizures: Unexplained seizures can be a symptom of neurological cancer.
- Cognitive changes: Memory loss, confusion, or difficulty concentrating may be present.
- Weakness or numbness: Patients may experience weakness or numbness in the limbs.[3]
Causes
The exact causes of neurological cancer are not fully understood. However, certain risk factors may increase the likelihood of developing this condition:
- Age: The risk of neurological cancer increases with age.
- Family history: Having a close relative with neurological cancer may increase the risk.
- Exposure to radiation: Previous exposure to radiation, such as radiation therapy for other conditions, may increase the risk.
- Genetic conditions: Certain genetic conditions, such as neurofibromatosis or Li-Fraumeni syndrome, may predispose individuals to neurological cancer.[4]
Diagnostic Steps
Medical History
A comprehensive medical history should be obtained to gather relevant patient information, including:
- Risk factors: Inquire about any family history of neurological cancer or exposure to radiation.
- Medical conditions: Determine if the patient has any pre-existing conditions that may increase the risk of neurological cancer.
- Symptoms: Assess the presence and duration of symptoms related to neurological cancer.[5]
Physical Examination
A thorough physical examination should be performed, focusing on specific signs or findings indicative of neurological cancer:
- Neurological examination: Assess the patient’s reflexes, muscle strength, coordination, and sensation.
- Visual examination: Evaluate the patient’s vision and eye movements.
- Balance and coordination assessment: Test the patient’s ability to walk, maintain balance, and perform coordinated movements.[6]
Laboratory Tests
Laboratory tests may be helpful in diagnosing neurological cancer. The following tests may be considered:
- Complete blood count (CBC): To assess for any abnormalities in blood cell counts.
- Blood chemistry panel: To evaluate organ function and detect any metabolic abnormalities.
- Tumor markers: Certain tumor markers, such as alpha-fetoprotein (AFP) or carcinoembryonic antigen (CEA), may be elevated in neurological cancer.[7]
Diagnostic Imaging
Imaging modalities play a crucial role in visualizing and assessing neurological cancer. The following imaging techniques may be used:
- Magnetic Resonance Imaging (MRI): Provides detailed images of the brain and spinal cord.
- Computed Tomography (CT) scan: Useful for detecting abnormalities in the brain and spinal cord.
- Positron Emission Tomography (PET) scan: Can help identify areas of increased metabolic activity, which may indicate the presence of cancer.[8]
Other Tests
Additional diagnostic tests may be necessary based on the clinical presentation. These may include:
- Lumbar puncture: To analyze cerebrospinal fluid for the presence of cancer cells or other abnormalities.
- Biopsy: A sample of tissue may be obtained for further analysis to confirm the presence of cancer.
- Genetic testing: In some cases, genetic testing may be performed to identify specific genetic mutations associated with neurological cancer.[9]
Follow-up and Patient Education
After the diagnostic steps have been completed, it is essential to provide appropriate follow-up care and patient education:
- Referral to a specialist: Depending on the diagnosis, the patient may be referred to a neurologist or oncologist for further management.
- Emotional support: Offer counseling or support groups to help patients cope with the fear and anxiety associated with neurological cancer.
- Education on treatment options: Provide information on available treatment options and their potential benefits and risks.
- Regular monitoring: Schedule regular follow-up appointments to monitor the patient’s condition and response to treatment.[10]
Possible Interventions
Traditional Interventions
Medications:
Top 5 drugs for neurological cancer:
- Temozolomide:
- Cost: $3,000-$5,000 per month.
- Contraindications: Hypersensitivity to temozolomide or its components.
- Side effects: Nausea, vomiting, fatigue.
- Severe side effects: Bone marrow suppression, liver toxicity.
- Drug interactions: None specified.
- Warning: Regular blood tests required to monitor blood cell counts.
- Carmustine:
- Cost: $2,000-$4,000 per month.
- Contraindications: Hypersensitivity to carmustine or its components.
- Side effects: Nausea, vomiting, hair loss.
- Severe side effects: Bone marrow suppression, lung toxicity.
- Drug interactions: None specified.
- Warning: Regular blood tests required to monitor blood cell counts.
- Lomustine:
- Cost: $1,500-$3,000 per month.
- Contraindications: Hypersensitivity to lomustine or its components.
- Side effects: Nausea, vomiting, fatigue.
- Severe side effects: Bone marrow suppression, liver toxicity.
- Drug interactions: None specified.
- Warning: Regular blood tests required to monitor blood cell counts.
- Bevacizumab:
- Cost: $5,000-$10,000 per month.
- Contraindications: Hypersensitivity to bevacizumab or its components.
- Side effects: High blood pressure, bleeding, proteinuria.
- Severe side effects: Gastrointestinal perforation, wound healing complications.
- Drug interactions: None specified.
- Warning: Increased risk of bleeding and blood clots.
- Temozolomide + Radiation Therapy:
- Cost: $10,000-$15,000 per month (including radiation therapy).
- Contraindications: Hypersensitivity to temozolomide or its components.
- Side effects: Nausea, vomiting, fatigue.
- Severe side effects: Bone marrow suppression, liver toxicity.
- Drug interactions: None specified.
- Warning: Regular blood tests required to monitor blood cell counts.
Surgical Procedures:
- Craniotomy: Surgical removal of brain tumors. Cost: $50,000-$100,000.
- Spinal cord surgery: Surgical removal of spinal cord tumors. Cost: $30,000-$70,000.
Alternative Interventions
- Acupuncture: May help alleviate symptoms and improve overall well-being. Cost: $60-$120 per session.
- Meditation and relaxation techniques: Can help reduce stress and anxiety. Cost: Free or minimal cost.
- Herbal supplements: Some herbs, such as turmeric or green tea extract, may have potential benefits in cancer treatment. Cost: Varies depending on the specific supplement.
- Mind-body therapies: Practices like yoga or tai chi may help improve physical and mental well-being. Cost: $10-$20 per session.
- Dietary modifications: A healthy diet rich in fruits, vegetables, and whole grains may support overall health. Cost: Varies depending on food choices.
Lifestyle Interventions
- Regular exercise: Engaging in physical activity can improve overall health and well-being. Cost: Free or minimal cost.
- Stress management techniques: Practices like deep breathing exercises or mindfulness can help reduce stress. Cost: Free or minimal cost.
- Adequate sleep: Getting enough sleep is essential for overall health and recovery. Cost: Free.
- Smoking cessation: Quitting smoking can improve treatment outcomes and overall health. Cost: Varies depending on the chosen method.
- Supportive care: Seeking emotional support from loved ones or joining support groups can help cope with the challenges of neurological cancer. Cost: Free or minimal cost.
It is important to note that the cost ranges provided are approximate and may vary depending on the location and availability of the interventions.
Mirari Cold Plasma Alternative Intervention
Understanding Mirari Cold Plasma
- Safe and Non-Invasive Treatment: Mirari Cold Plasma is a safe and non-invasive treatment option for various skin conditions. It does not require incisions, minimizing the risk of scarring, bleeding, or tissue damage.
- Efficient Extraction of Foreign Bodies: Mirari Cold Plasma facilitates the removal of foreign bodies from the skin by degrading and dissociating organic matter, allowing easier access and extraction.
- Pain Reduction and Comfort: Mirari Cold Plasma has a local analgesic effect, providing pain relief during the treatment, making it more comfortable for the patient.
- Reduced Risk of Infection: Mirari Cold Plasma has antimicrobial properties, effectively killing bacteria and reducing the risk of infection.
- Accelerated Healing and Minimal Scarring: Mirari Cold Plasma stimulates wound healing and tissue regeneration, reducing healing time and minimizing the formation of scars.
Mirari Cold Plasma Prescription
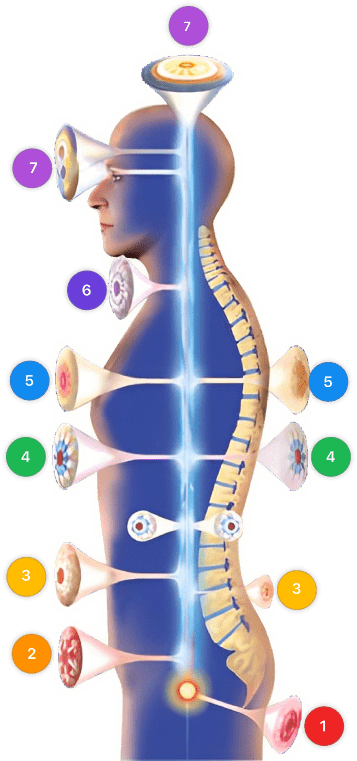
Video instructions for using Mirari Cold Plasma Device – N26 Fear cancer neurological system (ICD-10:Z71.1)
| Mild | Moderate | Severe |
| Mode setting: 7 (Immunotherapy) Location: 1 (Sacrum) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immunotherapy) Location: 1 (Sacrum) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immunotherapy) Location: 1 (Sacrum) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immunotherapy) Location: 4 (Heart, Bile & Pancreas) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 15 minutes, Evening: 15 minutes |
Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
Mode setting: 7 (Immunotherapy) Location: 7 (Neuro system & ENT) Morning: 30 minutes, Lunch: 30 minutes, Evening: 30 minutes |
| Total Morning: 60 minutes approx. $10 USD, Evening: 60 minutes approx. $10 USD |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
Total Morning: 120 minutes approx. $20 USD, Lunch: 120 minutes approx. $20 USD, Evening: 120 minutes approx. $20 USD, |
| Usual treatment for 7-60 days approx. $140 USD – $1200 USD | Usual treatment for 6-8 weeks approx. $2,520 USD – $3,360 USD |
Usual treatment for 3-6 months approx. $5,400 USD – $10,800 USD
|
 |
|
Use the Mirari Cold Plasma device to treat Fear cancer neurological system effectively.
WARNING: MIRARI COLD PLASMA IS DESIGNED FOR THE HUMAN BODY WITHOUT ANY ARTIFICIAL OR THIRD PARTY PRODUCTS. USE OF OTHER PRODUCTS IN COMBINATION WITH MIRARI COLD PLASMA MAY CAUSE UNPREDICTABLE EFFECTS, HARM OR INJURY. PLEASE CONSULT A MEDICAL PROFESSIONAL BEFORE COMBINING ANY OTHER PRODUCTS WITH USE OF MIRARI.
Step 1: Cleanse the Skin
- Start by cleaning the affected area of the skin with a gentle cleanser or mild soap and water. Gently pat the area dry with a clean towel.
Step 2: Prepare the Mirari Cold Plasma device
- Ensure that the Mirari Cold Plasma device is fully charged or has fresh batteries as per the manufacturer’s instructions. Make sure the device is clean and in good working condition.
- Switch on the Mirari device using the power button or by following the specific instructions provided with the device.
- Some Mirari devices may have adjustable settings for intensity or treatment duration. Follow the manufacturer’s instructions to select the appropriate settings based on your needs and the recommended guidelines.
Step 3: Apply the Device
- Place the Mirari device in direct contact with the affected area of the skin. Gently glide or hold the device over the skin surface, ensuring even coverage of the area experiencing.
- Slowly move the Mirari device in a circular motion or follow a specific pattern as indicated in the user manual. This helps ensure thorough treatment coverage.
Step 4: Monitor and Assess:
- Keep track of your progress and evaluate the effectiveness of the Mirari device in managing your Fear cancer neurological system. If you have any concerns or notice any adverse reactions, consult with your health care professional.
Note
This guide is for informational purposes only and should not replace the advice of a medical professional. Always consult with your healthcare provider or a qualified medical professional for personal advice, diagnosis, or treatment. Do not solely rely on the information presented here for decisions about your health. Use of this information is at your own risk. The authors of this guide, nor any associated entities or platforms, are not responsible for any potential adverse effects or outcomes based on the content.
Mirari Cold Plasma System Disclaimer
- Purpose: The Mirari Cold Plasma System is a Class 2 medical device designed for use by trained healthcare professionals. It is registered for use in Thailand and Vietnam. It is not intended for use outside of these locations.
- Informational Use: The content and information provided with the device are for educational and informational purposes only. They are not a substitute for professional medical advice or care.
- Variable Outcomes: While the device is approved for specific uses, individual outcomes can differ. We do not assert or guarantee specific medical outcomes.
- Consultation: Prior to utilizing the device or making decisions based on its content, it is essential to consult with a Certified Mirari Tele-Therapist and your medical healthcare provider regarding specific protocols.
- Liability: By using this device, users are acknowledging and accepting all potential risks. Neither the manufacturer nor the distributor will be held accountable for any adverse reactions, injuries, or damages stemming from its use.
- Geographical Availability: This device has received approval for designated purposes by the Thai and Vietnam FDA. As of now, outside of Thailand and Vietnam, the Mirari Cold Plasma System is not available for purchase or use.
References
- World Health Organization. (2021). International Classification of Primary Care, Second edition (ICPC-2). Retrieved from https://www.who.int/standards/classifications/other-classifications/international-classification-of-primary-care
- World Health Organization. (2021). International Classification of Diseases, 10th Revision (ICD-10). Retrieved from https://icd.who.int/browse10/2019/en
- American Brain Tumor Association. (2021). Brain Tumor Symptoms. Retrieved from https://www.abta.org/about-brain-tumors/brain-tumor-symptoms/
- National Cancer Institute. (2021). Risk Factors for Cancer. Retrieved from https://www.cancer.gov/about-cancer/causes-prevention/risk
- Mayo Clinic. (2021). Brain tumor diagnosis. Retrieved from https://www.mayoclinic.org/diseases-conditions/brain-tumor/diagnosis-treatment/drc-20350088
- National Institute of Neurological Disorders and Stroke. (2021). Neurological Diagnostic Tests and Procedures. Retrieved from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Neurological-Diagnostic-Tests-and-Procedures-Fact
- American Association for Clinical Chemistry. (2021). Tumor Markers. Retrieved from https://labtestsonline.org/tests/tumor-markers
- Radiological Society of North America. (2021). Brain Tumor Imaging. Retrieved from https://www.radiologyinfo.org/en/info.cfm?pg=braintumor
- National Cancer Institute. (2021). Genetic Testing for Inherited Cancer Susceptibility Syndromes. Retrieved from https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
- American Society of Clinical Oncology. (2021). Follow-up Care for Cancer. Retrieved from https://www.cancer.net/survivorship/follow-care-cancer
Related articles
Made in USA





























