The MIRARI® Cold Plasma System represents a revolutionary breakthrough in medical technology. As the world’s first handheld cold atmospheric plasma (CAP) device, this innovative system has transformed how healthcare professionals approach wound healing, pain management, and dermatological treatments.
Developed and patented by General Vibronics Inc. in the USA, the MIRARI system has evolved through multiple generations. Each iteration brings enhanced clinical performance, extended application duration, and greater user convenience and safety.
But what exactly sets each generation apart? And how do these technical specifications impact real-world clinical outcomes?
The Evolution of MIRARI® Generations: A Game-Changing Journey
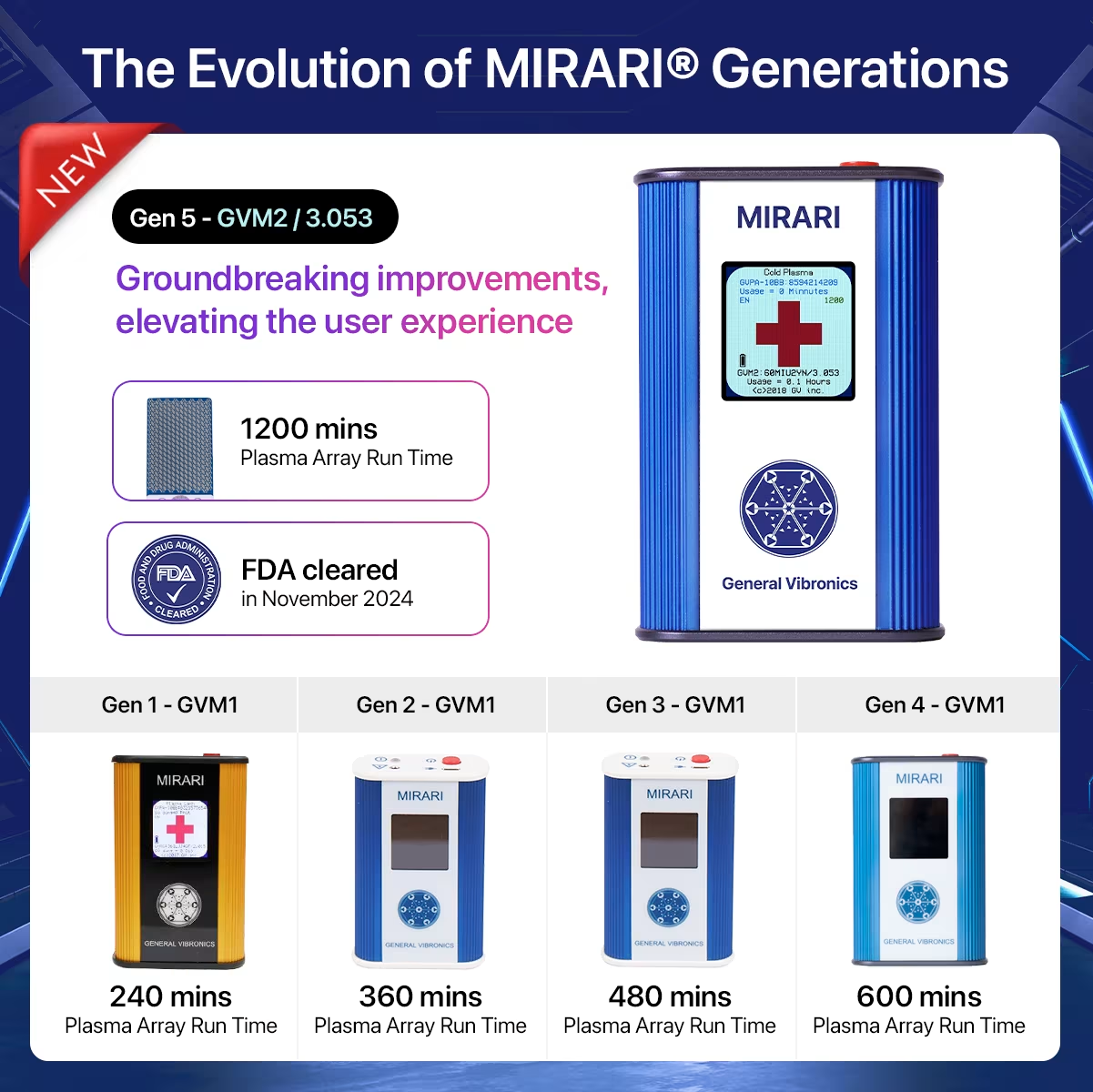
Device Evolution by Application Duration
The MIRARI Cold Plasma System has undergone remarkable transformation across five distinct generations. Each version addresses specific clinical needs while building upon proven plasma therapy foundations.
| Generation | Application Duration | Primary Use | Key Innovation |
| MIRARI 240 min | 240 minutes | Clinical research (basic dermatology) | Foundation CAP technology |
| MIRARI 360 min | 360 minutes | Research-focused with improved durability | Enhanced battery technology |
| MIRARI 480 min | 480 minutes | Clinics and outpatient therapy | Dual treatment modalities |
| MIRARI 600 min | 600 minutes | Clinics & hospitals, modernized design | Ergonomic improvements |
| MIRARI 1200 min (FDA) | 1200 minutes | Full hospital-grade, multi-specialty use | FDA clearance & full compliance |
Regulatory Milestones That Changed Everything
The MIRARI 1200 version achieved a historic milestone. It received FDA clearance – a testament to its safety, performance, and efficacy.
This certification validates the system’s effectiveness in wound healing and dermatological applications. More importantly, it opens doors for widespread clinical adoption across hospital systems and specialized medical centers.
Technical Specifications by Generation: The Engineering Marvel
Core Components That Power Every Version
Every MIRARI Cold Plasma System shares fundamental components that deliver consistent therapeutic results:
- Plasma Driver: The heart of the system. This rechargeable control box houses the RF/DBD plasma generator that creates therapeutic plasma fields.
- Plasma Array: An electrode-based CAP applicator measuring 2.50″ x 1.72″. This interchangeable component generates thousands of tiny plasma plumes on a printed circuit board surface.
Insulating Pouches:
- Waterproof ePTFE pouch for moisture protection
- Thermal-regulating fleece pouch for temperature control
Power Source: Modern USB-C charging with battery capacity optimized for each generation’s runtime requirements.
Operating Characteristics: Precision Engineering at Work
| Parameter | Value/Description | Clinical Impact |
| RF Frequency | 80 kHz (monopolar) | Optimal tissue penetration |
| Plasma Generation Method | Dielectric Barrier Discharge (DBD) | Safe, non-thermal plasma creation |
| Output Voltage (Peak-to-Peak) | >700V required for CAP generation | Sufficient ionization for therapeutic effects |
| Thermal Limit | Max surface temperature: 43°C | Patient comfort and safety |
| UV Emission | >99% UV-A spectrum (310–470 nm) | Antimicrobial effects without harmful radiation |
| Ozone Emission | <40 ppb (well below UL 867 limit) | Environmental safety compliance |
Safety Features: Protection at Every Level
The MIRARI system incorporates multiple safety mechanisms:
- Auto shutdown for short circuits, overheating, or arcing
- Capacitive & resistive heat modulation based on tissue type
- Integrated sensors and temperature feedback control
These features ensure patient safety while maintaining therapeutic efficacy across all treatment protocols.
Distinctive Features Across Versions: What Sets Each Generation Apart
| Feature | 240 | 360 | 480 | 600 | 1200 |
| Primary Use | Research | Research | Clinics | Clinics/Hospitals | Hospitals/Certified use |
| Hardware Upgrades | Basic CAP array | Enhanced battery | Improved design | Ergonomic improvements | FDA-approved specs |
| Operating Duration | 240 mins | 360 mins | 480 mins | 600 mins | 1200 mins |
| Target User | Researchers | Researchers | Physicians | Clinics | Hospitals |
| Regulatory Readiness | Pre-market | Pre-market | Clinical-grade | CE-ready | FDA Cleared |
| System Safety & Feedback | Basic | Moderate | Advanced | Advanced | Full compliance suite |
Engineering and Functional Innovations: The Science Behind Success
Plasma Modulation Technology
The MIRARI system employs sophisticated plasma modulation techniques:
- Duty-cycle controlled RF output operates at 30-40% to limit heat generation. This precise control ensures therapeutic effectiveness without thermal damage to healthy tissue.
- Custom resonance tuning per array configuration optimizes plasma generation for specific treatment protocols. This innovation maximizes therapeutic outcomes while maintaining safety standards.
Dual Treatment Modalities: Versatility in Action
Advanced versions (480+ models) feature dual treatment modalities:
- CAP Mode: Designed for superficial, high-water-content tissue. Perfect for wound healing, skin rejuvenation, and surface lesion treatment.
- RES Mode: Targets deep, low-hydration tissue with resistive heating. Ideal for musculoskeletal conditions, deep tissue pain, and chronic inflammatory conditions.
Treatment Protocols: Precision in Practice
Standardized protocols ensure optimal outcomes:
- Maximum treatment time: 15 minutes per session
- Minimum interval: 30 minutes between sessions
- Treatment frequency: Customized based on condition severity and patient response
Clinical and Application Differentiation: Real-World Impact
Earlier Versions: Building the Foundation
MIRARI 240 and 360 versions established the foundation for cold plasma therapy. These systems enabled:
- Basic dermatology research
- Small-scale clinical trials
- Proof-of-concept studies
Research using these early versions demonstrated wound healing acceleration, pain reduction, and antimicrobial effects that paved the way for advanced clinical applications.
Advanced Versions: Transforming Patient Care
MIRARI 480, 600, and 1200 versions revolutionized clinical practice with expanded indications:
Wound Healing Applications:
- Acute and chronic ulcers
- Diabetic foot wounds
- Pressure ulcers
- Post-surgical healing
Dermatological Treatments:
- Skin lesion removal
- Acne treatment
- Scar reduction
- Skin rejuvenation
Musculoskeletal Conditions:
- Rheumatoid arthritis (RA)
- Osteoarthritis (OA)
- Fibromyalgia
- Sports injuries
Pain Management:
- Chronic pain syndromes
- Neuropathic pain
- Inflammatory pain
- Post-operative pain
Integrated Benefits in Later Models
Later MIRARI generations offer significant improvements:
- Enhanced Ergonomics: Improved handheld design reduces operator fatigue during extended treatment sessions.
- Automatic Energy Optimization: Smart algorithms adjust energy profiles based on tissue type and treatment objectives.
- Advanced Clinical Feedback: Real-time monitoring provides treatment data and safety alerts for optimal patient outcomes.
The Clinical Evidence: Proven Results Across Generations
Research Breakthrough Moments
Clinical studies using various MIRARI generations have demonstrated remarkable results:
- Wound Healing Studies: Patients treated with cold plasma therapy showed 92% wound closure rates compared to 32% in control groups.
- Pain Management Research: Fibromyalgia patients experienced significant pain reduction with cold plasma treatment, with effects lasting weeks after treatment completion.
- Dermatological Applications: Acne treatment studies showed significant improvement in lesion count and inflammation markers compared to conventional therapies.
Safety Profile: Consistently Excellent
Across all generations, the MIRARI Cold Plasma System maintains an excellent safety profile:
- Zero serious adverse events reported in clinical trials
- Minimal side effects – occasional mild skin redness
- No contraindications for most patient populations
- Compatible with existing treatment protocols
Future Implications: What’s Next for Cold Plasma Technology?
Emerging Applications
The MIRARI system’s evolution continues with research into:
- Neurological Applications: Early studies suggest potential for neuropathic pain and nerve regeneration.
- Oncological Support: Investigation into adjuvant cancer care and chemotherapy side effect management.
- Aesthetic Medicine: Advanced skin rejuvenation and anti-aging applications.
Technology Integration
Future MIRARI generations may incorporate:
- AI-Powered Treatment Planning: Machine learning algorithms for personalized treatment protocols.
- Telemedicine Integration: Remote monitoring and treatment adjustment capabilities.
- Enhanced Portability: Miniaturized components for point-of-care applications.
Conclusion: A Legacy of Innovation and Clinical Excellence
The MIRARI® Cold Plasma System represents more than technological advancement. It embodies a commitment to patient care, clinical excellence, and medical innovation.
Each generation reflects continuous research and development focused on clinical utility, safety, and regulatory compliance. The 1200-minute FDA-cleared version integrates all technological, ergonomic, and safety refinements developed over years of clinical experience.
This progressive design evolution has enabled successful adaptation from research laboratory settings to frontline hospital use. Healthcare providers worldwide now have access to proven cold plasma therapy that delivers consistent, safe, and effective patient outcomes.
The MIRARI Cold Plasma System continues to set the standard for non-invasive medical technology. As we look toward the future, the foundation built by these five generations will undoubtedly support even more innovative applications and improved patient care.
Key Takeaways:
- Five distinct generations each serve specific clinical needs
- Progressive improvements in durability, safety, and efficacy
- FDA clearance validates clinical effectiveness and safety
- Dual treatment modalities maximize therapeutic versatility
- Excellent safety profile across all clinical applications
- Proven clinical outcomes in wound healing, pain management, and dermatology
The MIRARI Cold Plasma System stands as a testament to what’s possible when innovative engineering meets clinical excellence. Each generation has contributed to a comprehensive solution that addresses real-world healthcare challenges with precision, safety, and remarkable effectiveness.
Related articles
Made in USA