Cold plasma in cancer therapy represents one of the most promising breakthroughs in modern oncology, offering a selective, non-invasive approach that targets malignant cells while preserving healthy tissue. This innovative technology harnesses reactive oxygen and nitrogen species (RONS) to induce cancer cell death through multiple mechanisms, providing hope for patients facing treatment-resistant tumors and those seeking alternatives to traditional therapies with severe side effects.
Understanding Cold Atmospheric Plasma Technology
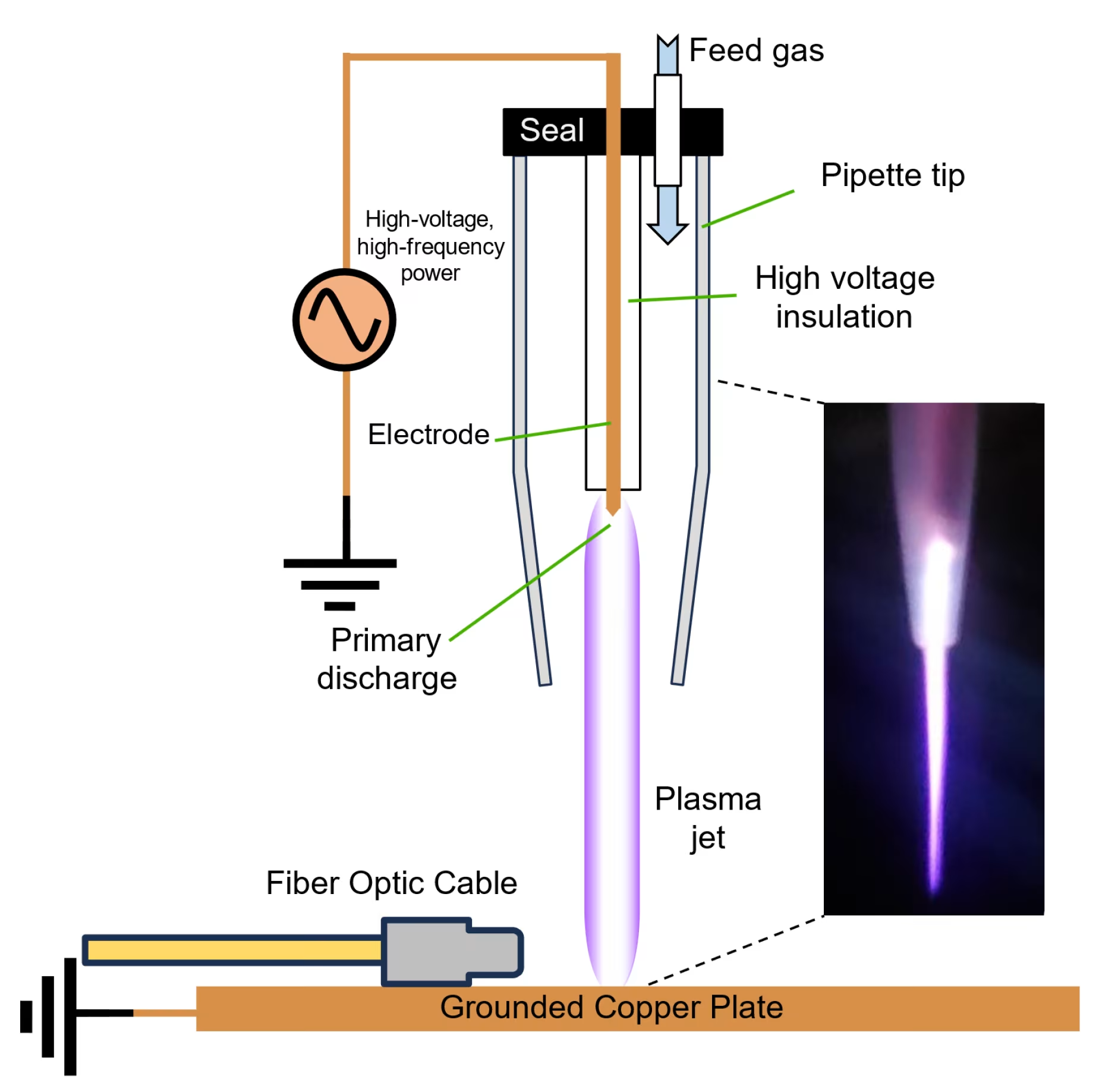
Cold atmospheric plasma (CAP) operates at near room temperature, distinguishing it from thermal plasma used in industrial applications. This fourth state of matter contains a complex mixture of ions, electrons, neutral particles, and electromagnetic fields that generate biologically active species when applied to tissues[1].
Scientific Mechanisms Behind Cold Plasma
The therapeutic power of cold plasma in cancer lies in its ability to produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) that selectively target malignant cells. Cancer cells exhibit increased membrane permeability and elevated baseline oxidative stress compared to normal cells, making them more vulnerable to additional ROS burden[2].
Nitric Oxide vs Reactive Oxygen Species Pathways
Traditional cold plasma devices primarily generate ROS through atmospheric ionization, while newer technologies like the Mirari Cold Plasma system utilize nitric oxide (NO) as the primary therapeutic agent. Developed by General Vibronics and commercialized by Mirari Doctor, this innovative approach offers enhanced precision in targeting cancer cells through NO’s unique signaling properties[3].
Selective Cancer Cell Targeting Mechanisms
Cold plasma in cancer treatment demonstrates remarkable selectivity through several key mechanisms that exploit fundamental differences between malignant and healthy cells.
Enhanced Membrane Permeability in Cancer Cells
Cancer cells express higher levels of aquaporins on their cytoplasmic membranes, facilitating faster uptake of hydrogen peroxide and other reactive species generated by cold plasma treatment. This increased permeability allows therapeutic agents to penetrate cancer cells more effectively than normal cells[4].
Catalase Inactivation and Self-Enhancing RONS Loop
Cold plasma initially inactivates membrane-associated catalase on tumor cells, creating a self-propagating cycle where extracellular hydrogen peroxide and peroxynitrite penetrate cells, deplete glutathione reserves, and drive lipid peroxidation. This cascade ultimately triggers apoptosis through HOCl signaling pathways[1].
Clinical Evidence and FDA-Approved Trials
The first FDA-approved Phase I clinical trial for cold plasma in cancer treatment has provided groundbreaking evidence of safety and efficacy in treating advanced solid tumors.
Canady Helios Cold Plasma Clinical Results
Between March 2020 and April 2021, twenty patients with stage IV metastatic or recurrent solid tumors received intraoperative cold plasma treatment following surgical resection. The trial demonstrated exceptional safety with no device-related adverse events and promising survival outcomes[5].
Survival and Recurrence Outcomes
For patients achieving R0 resection (complete tumor removal), the local non-recurrence probability at 28 months was 75%, while the cumulative overall survival rate reached 24% at 31 months. These results represent significant improvements over historical controls for similar patient populations[5].
Combination Therapy Benefits
Recent studies have shown that cold plasma in cancer treatment achieves enhanced efficacy when combined with conventional therapies. A lymphosarcoma study demonstrated that combining cold plasma with doxorubicin hydrochloride resulted in a threefold reduction in tumor volume compared to chemotherapy alone[6].
Technical Specifications and Treatment Parameters
| Parameter | Specification | Clinical Significance |
|---|---|---|
| Operating Temperature | 26-30°C | Prevents thermal tissue damage |
| Plasma Generation | Dielectric Barrier Discharge | Stable, controlled plasma formation |
| Treatment Duration | 5-15 minutes per session | Optimized for maximum efficacy |
| Penetration Depth | 3-8 mm | Targets superficial and deep tissue layers |
| ROS/RNS Production | 10^15-10^17 particles/cm³ | Therapeutic threshold for cancer cell death |
| Electromagnetic Field | 80 kHz monopolar RF | Enhanced cellular membrane permeability |
Clinical Applications Across Cancer Types
Cold plasma in cancer therapy has demonstrated efficacy across multiple tumor types, with varying mechanisms of action depending on cancer cell characteristics.
Skin Cancer and Melanoma Treatment
Melanoma cells show particular sensitivity to cold plasma treatment due to their high metabolic activity and increased ROS production. Studies combining cold plasma with gold nanoparticles have shown enhanced selective cytotoxicity against melanoma cells while sparing healthy fibroblasts[7].
Breast Cancer Applications
Triple-negative breast cancer, known for its aggressive nature and limited treatment options, has shown promising responses to cold plasma therapy. The 4T1 mouse model demonstrated significant tumor volume reduction and improved survival rates with both helium and argon plasma treatments[8].
Gastrointestinal and Lung Cancers
Cold plasma treatment has shown effectiveness against various solid tumors including colorectal, lung, and gastric cancers. The selective targeting mechanism appears particularly effective in rapidly dividing cancer cells characteristic of these tumor types[9].
Safety Profile and Risk Assessment
| Safety Parameter | Clinical Finding | Implication |
|---|---|---|
| Thermal Effects | No temperature elevation >45°C | Safe for all tissue types |
| Systemic Toxicity | No adverse systemic effects reported | Suitable for immunocompromised patients |
| Local Tissue Damage | Selective cancer cell targeting | Preserves healthy tissue integrity |
| Treatment Tolerance | Well-tolerated across age groups | Applicable to elderly and frail patients |
| Drug Interactions | No known contraindications | Compatible with existing therapies |
Immunogenic Cell Death and Immune System Activation
Cold plasma in cancer treatment induces immunogenic cell death (ICD), releasing damage-associated molecular patterns (DAMPs) that activate dendritic cells and promote T-cell priming. This immune activation creates systemic anti-tumor responses that may prevent metastasis and recurrence[10].
Comparing Cold Plasma to Traditional Cancer Therapies
Unlike chemotherapy and radiation therapy, cold plasma offers targeted treatment without systemic toxicity. The technology’s selectivity for cancer cells addresses one of oncology’s greatest challenges: achieving therapeutic efficacy while minimizing damage to healthy tissues.
Advantages Over Conventional Treatments
Cold plasma therapy provides several distinct advantages including absence of drug resistance, minimal side effects, and compatibility with existing treatment protocols. The technology can be applied intraoperatively to treat surgical margins or used as adjuvant therapy to enhance conventional treatments[11].
Future Directions and Emerging Applications
Research continues to expand cold plasma applications in cancer treatment, with particular focus on combination therapies and personalized treatment protocols.
Nanoparticle Enhancement Strategies
Combining cold plasma with targeted nanoparticles represents a promising frontier in precision cancer therapy. Studies show that plasma treatment enhances nanoparticle uptake specifically in cancer cells, potentially improving drug delivery and therapeutic outcomes[7].
Immunotherapy Synergy
The immunomodulatory effects of cold plasma make it an ideal candidate for combination with checkpoint inhibitors and other immunotherapies. Early research suggests that plasma-induced immune activation may overcome resistance to immunotherapy in previously unresponsive tumors[10].
Device Innovation and Accessibility
The Mirari Cold Plasma system represents a significant advancement in making cold plasma technology accessible for clinical use. This handheld device, available through Mirari Doctor (miraridoctor.com), utilizes proprietary nitric oxide generation to deliver precise, controlled plasma therapy in various clinical settings[3].
Standardization and Treatment Protocols
As cold plasma in cancer treatment moves toward widespread clinical adoption, standardization of treatment parameters becomes crucial. Research focuses on optimizing exposure times, plasma composition, and treatment schedules for different cancer types to ensure reproducible clinical outcomes[12].
Patient Selection and Treatment Planning
Successful cold plasma therapy requires careful patient selection and individualized treatment planning. Factors including tumor type, stage, location, and patient health status all influence treatment outcomes and protocol selection.
Optimal Candidate Identification
Patients with localized tumors, surgical candidates requiring margin treatment, and those seeking adjuvant therapy represent ideal candidates for cold plasma treatment. The technology’s safety profile makes it particularly suitable for patients who cannot tolerate conventional therapies[5].
Frequently Asked Questions
Is cold plasma treatment safe for all cancer patients?
Cold plasma therapy demonstrates an excellent safety profile with no reported systemic adverse effects in clinical trials. The treatment operates at body temperature and selectively targets cancer cells, making it suitable for most patients including those with compromised immune systems.
How does cold plasma compare to chemotherapy in terms of side effects?
Unlike chemotherapy, cold plasma treatment produces no systemic toxicity, hair loss, nausea, or immune suppression. The selective targeting mechanism preserves healthy tissue while effectively eliminating cancer cells, resulting in minimal side effects.
Can cold plasma be used alongside other cancer treatments?
Yes, cold plasma therapy shows excellent compatibility with existing cancer treatments. Studies demonstrate enhanced efficacy when combined with chemotherapy, radiation, and surgical resection, often improving outcomes while reducing overall treatment toxicity.
How long does each cold plasma treatment session take?
Treatment sessions typically last 5-15 minutes depending on tumor size and location. The non-invasive nature of the therapy allows for outpatient treatment with immediate return to normal activities.
What types of cancer respond best to cold plasma therapy?
Research shows effectiveness across multiple cancer types including melanoma, breast cancer, lung cancer, and gastrointestinal tumors. Rapidly dividing cancer cells and those with high metabolic activity tend to show the greatest response to cold plasma treatment.
Conclusion
Cold plasma in cancer treatment represents a paradigm shift in oncological care, offering selective, safe, and effective therapy for patients facing challenging diagnoses. The technology’s unique mechanism of action, demonstrated clinical efficacy, and excellent safety profile position it as a valuable addition to the cancer treatment arsenal. As research continues to refine treatment protocols and expand applications, cold plasma therapy promises to improve outcomes for cancer patients while reducing the burden of treatment-related side effects that have long plagued conventional therapies.
References
- Frontiers in Oncology. (2023). Adjuvant composite cold atmospheric plasma therapy increases treatment efficacy in lymphosarcoma. https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2023.1171042/full
- MDPI Applied Sciences. (2024). Studies of Applications of Cold Plasma Systems in Cancer Treatment. https://www.mdpi.com/2673-7140/4/4/60
- Mirari Doctor. (2024). Cold Plasma in Cancer: A Future Method. https://miraridoctor.com/cold-plasma-in-cancer/
- Nature Scientific Reports. (2025). Cold plasma enhances the generation of reactive oxygen species and gold nanoparticle delivery to melanoma cells. https://pubmed.ncbi.nlm.nih.gov/40224200/
- PMC. (2023). The First Cold Atmospheric Plasma Phase I Clinical Trial for the treatment of advanced solid tumors. https://pmc.ncbi.nlm.nih.gov/articles/PMC10378184/
- Frontiers in Oncology. (2023). Adjuvant composite cold atmospheric plasma therapy increases treatment efficacy. https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2023.1171042/full
- PubMed. (2025). Cold plasma enhances the generation of reactive oxygen species. https://pubmed.ncbi.nlm.nih.gov/40224200/
- Nature Scientific Reports. (2025). Helium and argon cold plasma effects on the 4T1 cancer cells. https://www.nature.com/articles/s41598-025-95065-z
- PMC. (2022). Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor. https://pmc.ncbi.nlm.nih.gov/articles/PMC9139675/
- PMC. (2021). Cold Physical Plasma in Cancer Therapy: Mechanisms, Signaling. https://pmc.ncbi.nlm.nih.gov/articles/PMC8906949/
- PMC. (2025). Cold Atmospheric Plasma in Oncology: A Review and Perspectives. https://pmc.ncbi.nlm.nih.gov/articles/PMC11987927/
- Trends in Sciences. (2024). Cold Atmospheric Plasma in Cancer Therapy. https://tis.wu.ac.th/index.php/tis/article/view/8753
Related articles
Made in USA