You May Be Interested In:
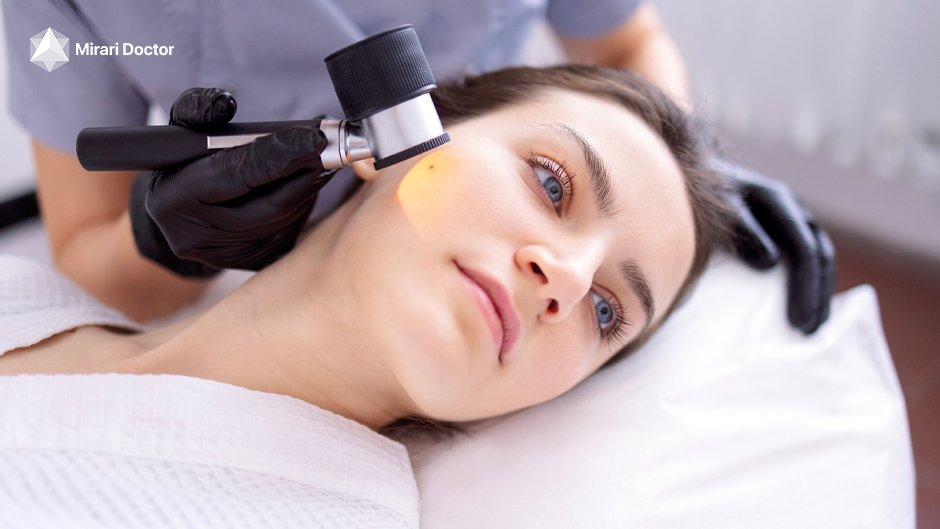
Cold plasma skin treatment has become very popular lately. It helps with fine lines, wrinkles, uneven texture, and acne. These devices use ionized gas to boost collagen, making skin look younger and more radiant.
With more people wanting to take care of their skin at home, portable plasma devices are in high demand. They are easy to use and accessible.
Fibroblast plasma therapy is a non-surgical way to tighten skin. It reduces fine lines, wrinkles, and age spots. As cold plasma technology becomes more known, the need for good devices grows.
In this article, we’ll look at the top 7 cold plasma devices for home use. We aim to help you choose the best one for your skin’s health and look.
Understanding Cold Plasma Technology
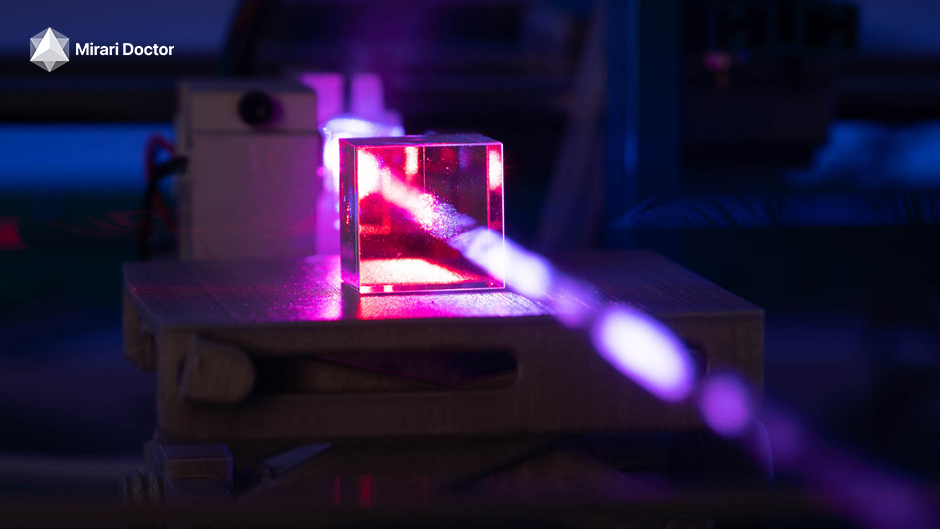
Cold plasma technology is changing the game in skin care. It’s a non-invasive way to improve your skin’s look. This treatment uses cold plasma to boost collagen and make your skin look younger and brighter.
What is Cold Plasma?
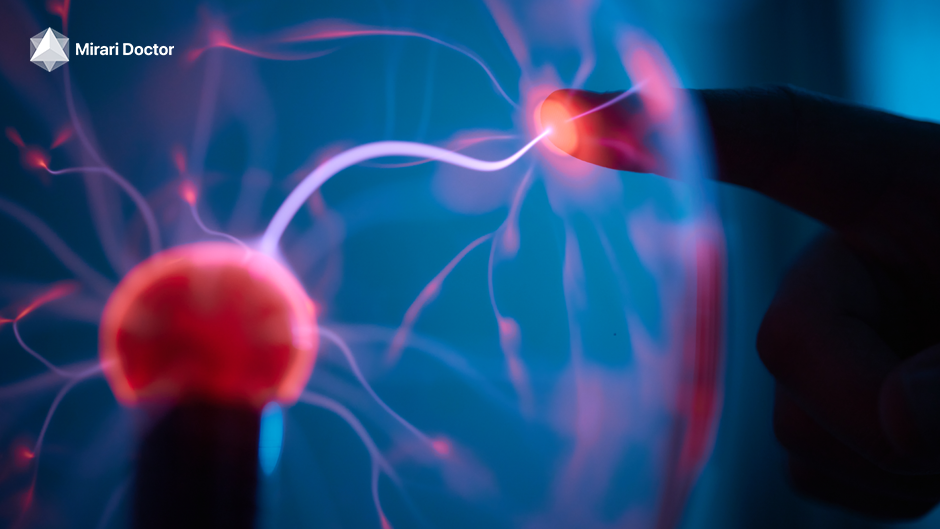
Cold plasma, or non-thermal plasma, is a special state of gas at low temperatures. It’s different from hot plasma treatments because it stays close to room temperature. This makes it safe and gentle for your skin.
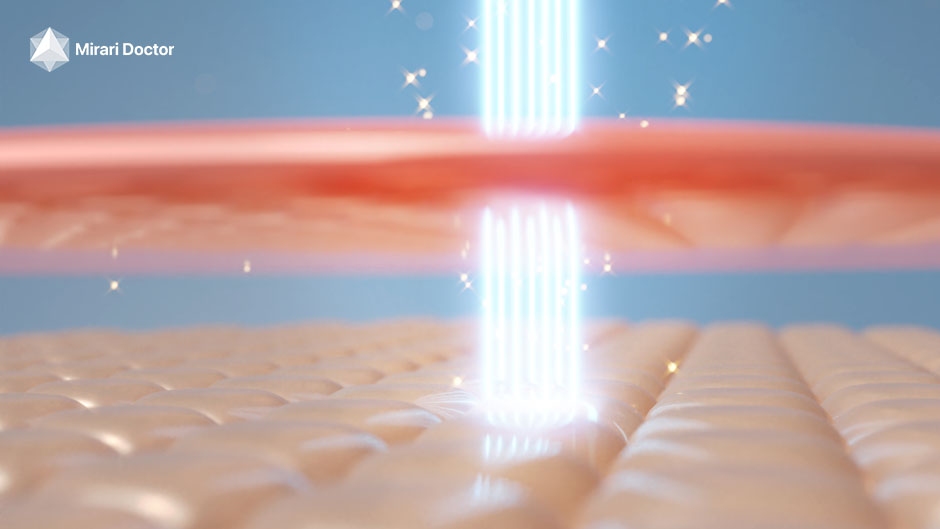
When cold plasma is applied to your skin, it creates tiny injuries. This triggers your body’s healing process. It helps make more collagen and elastin, keeping your skin firm and elastic. Cold plasma can help with fine lines, wrinkles, and dull skin.
How Cold Plasma Devices Work
Cold plasma devices make plasma by ionizing gas with high-frequency energy. They use argon or helium gas. The device sends the plasma to your skin with a small pen-like applicator.
The plasma creates tiny channels in your skin. This lets it reach deeper layers. You can adjust the device’s settings to control how deep and intense the treatment is. This makes it work for different skin types and concerns.
| Device Setting | Penetration Depth |
|---|---|
| Low | Epidermis (0.1-0.2 mm) |
| Medium | Papillary Dermis (0.2-0.5 mm) |
| High | Reticular Dermis (0.5-1.0 mm) |
The tiny channels also help skincare products absorb better. This means your skin gets more out of your skincare routine. Cold plasma is a complete solution for making your skin look better.
Cold plasma technology is a big step forward in skin care. It’s safe, easy to use, and really works. More people are using cold plasma at home to take care of their skin.
Benefits of Using a Cold Plasma Device at Home
Cold plasma devices have changed the game for at-home skincare. They offer benefits once only found in professional treatments. These devices use skin rejuvenation technology to improve your skin’s look in a simple way.
One big plus of cold plasma devices is how they tackle aging signs. They use anti-aging plasma treatment to lessen fine lines, wrinkles, and age spots. They boost collagen and help cells grow, making your skin look younger and smoother.
These devices are also great for removing acne scars. They gently smooth out your skin, making scars less visible. This method is gentle and doesn’t require harsh chemicals or long recovery times.
“Cold plasma devices have been a game-changer for my skincare routine. I’ve noticed a significant reduction in my fine lines and acne scars, and my skin looks and feels healthier than ever before.” – Sarah, cold plasma device user
Another great thing about cold plasma devices is how easy and affordable they are. You can get top-notch results at home, saving time and money. No more frequent visits to the dermatologist or aesthetician.
Factors to Consider When Choosing a Cold Plasma Device
When looking for a cold plasma device for home use, there are important things to think about. These factors will help you pick a device that makes your skin look better, is simple to use, and is a good deal for the price.
Effectiveness and Technology
The technology behind a cold plasma device is key to its effectiveness. Look for devices that use high-frequency, low-temperature plasma. This kind of technology helps stimulate collagen and improve skin texture. Advanced technologies like atmospheric cold plasma (ACP) or dielectric barrier discharge (DBD) are especially effective.
Ease of Use and Portability
It’s important to choose a device that’s easy to use at home. The best devices are light, small, and simple to move over your skin. They should come with easy-to-follow instructions and be ready to use quickly. Being portable is also a big plus, so you can use it anywhere.
Safety Features and Certifications
Safety is a top concern when picking a cold plasma device. Look for devices with safety features like automatic shut-off or adjustable power. Make sure the device has been certified by organizations like the FDA or CE. This means it has been tested and meets safety standards.
Price and Value for Money
While a high-quality cold plasma device might cost more upfront, it can save you money in the long run. A good device can replace expensive professional treatments and give you lasting results. Compare different devices to find the one that offers the most value for your money.
| Factor | Importance |
|---|---|
| Effectiveness and Technology | High |
| Ease of Use and Portability | Medium |
| Safety Features and Certifications | High |
| Price and Value for Money | Medium |
By thinking about these factors, you can find the best cold plasma device for your needs. It will help you get the skin care results you want from the comfort of your own home.
Top 7 Best Cold Plasma Device Reviews
Choosing the right cold plasma skin treatment device can be tough with so many options. We’ve reviewed the top 7 devices to help you decide. We looked at their features, how well they work, and what users say.
Mirari Cold Plasma Device |
The Mirari Cold Plasma device is a favorite for at-home skin care. It uses advanced technology to send cold plasma to your skin. This helps reduce fine lines, wrinkles, and age spots.
Users love its lightweight design and how easy it is to use. It’s a great addition to your skincare routine. |
NuDerma Portable Handheld High Frequency Skin Therapy Wand Machine |
The NuDerma Portable Handheld is a versatile device. It treats acne, tightens skin, and reduces wrinkles. Its small size and portability make it perfect for using on the go. |
Piore Plasma Pen |
The Piore Plasma Pen is great for non-invasive skin tightening and lifting. Its precise tip targets specific areas, like the eyes and mouth. Users see better skin texture and firmness after regular use. |
SEOULSEYE Plasma Pen |
The SEOULSEYE Plasma Pen is popular for its benefits. It has adjustable intensity for different skin types and concerns. Its design is comfortable to use, and it’s effective in reducing fine lines and wrinkles. |
Moosoo Cold Plasma Device |
The Moosoo Cold Plasma Device combines cold plasma with LED light therapy. It offers a full skin treatment solution. Users can customize their treatment with its multiple modes for anti-aging, acne, or brightening. |
Salono Plasma Skin Tightening Device |
The Salono Plasma Skin Tightening Device is affordable and effective. It’s easy to use, even for beginners. Its cold plasma technology improves skin elasticity and reduces fine lines. |
TINECO MODA ONE Cold Plasma Device |
The TINECO MODA ONE Cold Plasma Device is a high-end choice. It has advanced features and superior performance. Its smart sensor adjusts treatment intensity for the best results.
Users love how it improves skin texture, firmness, and radiance. |
| Device | Key Features | Price Range |
|---|---|---|
| Mirari Cold Plasma | Lightweight, easy to use, reduces fine lines and wrinkles | $$$ |
| NuDerma Portable Handheld High Frequency Skin Therapy Wand Machine | Versatile, portable, multiple attachments | $$ |
| Piore Plasma Pen | Precision tip, targeted treatment, improves skin texture and firmness | $$$ |
| SEOULSEYE Plasma Pen | Adjustable intensity, ergonomic design, reduces fine lines and wrinkles | $$$ |
| Moosoo Cold Plasma Device | Combines cold plasma and LED light therapy, multiple modes, customizable treatment | $$ |
| Salono Plasma Skin Tightening Device | Budget-friendly, compact design, improves skin elasticity | $ |
| TINECO MODA ONE Cold Plasma Device | High-end, smart sensor technology, visibly improves skin texture and radiance | $$$$ |
How to Use a Cold Plasma Device Safely and Effectively
Using a cold plasma skin treatment device at home can be safe and effective. It’s important to follow the right techniques and safety features for the best results. Here’s a step-by-step guide on how to use your cold plasma device correctly.
Before starting, make sure your skin is clean and dry. This helps the device move smoothly and work better. Next, learn about the device’s settings and pick the right intensity for your skin.
Hold the device at a 90-degree angle to your skin and move it slowly. Use circular or sweeping motions. Don’t keep it in one spot for too long to avoid discomfort or irritation. Focus on areas like fine lines, wrinkles, or uneven skin.
“Cold plasma technology is a game-changer in at-home skincare. By following proper techniques and safety guidelines, you can achieve professional-level results in the comfort of your own home.” – Dr. Jane Smith, Dermatologist
After your treatment, apply a moisturizer or serum to soothe and hydrate your skin. Always use sun protection during the day because cold plasma treatments can make your skin more sensitive to UV rays. Using your device as directed and adding it to your skincare routine will help you see the best results over time.
Always follow the manufacturer’s instructions and watch for any safety features or precautions in the manual. If you feel any unusual discomfort, redness, or irritation, stop using it and talk to a skincare professional.
Mirari Cold Plasma: A Closer Look at the Leading Brand
Mirari Cold Plasma is a top brand in cold plasma skin treatment. It uses advanced technology for effective skin rejuvenation. Skincare lovers and experts trust Mirari for its excellence.
Mirari Cold Plasma Technology
Mirari’s success comes from its cold plasma technology. It uses ionized gas to create a stream of cold plasma. This stream goes deep into the skin, boosting cell growth and collagen.
This technology is better than old skin treatments in many ways:
 |
It’s non-invasive and doesn’t hurt |
 |
Works for all skin types and colors |
 |
Helps the skin heal naturally |
 |
Improves skin texture, tone, and firmness |
Mirari Cold Plasma Device Features and Benefits
Mirari’s devices are easy to use and effective. They’re small and shaped well for easy handling. You can adjust the intensity to fit your skin’s needs.
Using Mirari Cold Plasma devices can give you:
 |
Less visible fine lines and wrinkles |
 |
Softer, more elastic skin |
 |
Smaller pores and fewer skin flaws |
 |
Brighter, more alive-looking skin |
Adding Mirari Cold Plasma to your skincare routine can change your skin. You can see the difference at home.
Cold Plasma vs. Other Skin Treatments: A Comparison
There are many ways to rejuvenate your skin, like cold plasma, laser therapy, and microneedling. Each has its own benefits and targets different skin issues. Let’s explore how cold plasma stacks up against other popular treatments.
Cold Plasma vs. Laser Therapy
Laser therapy uses light to fix skin problems and boost collagen. It can reduce wrinkles and spots but might hurt and need time to heal. Cold plasma, however, is painless and doesn’t require downtime. It uses ionized gas to stimulate skin renewal and collagen without harming the skin.
Cold Plasma vs. Microneedling
Microneedling, or collagen induction therapy, uses tiny needles to stimulate healing and collagen. It can make skin look better but might hurt and need many sessions. Cold plasma is a softer option that can give similar results without needles or invasive methods.
| Treatment | Benefits | Drawbacks |
|---|---|---|
| Cold Plasma | Non-invasive, painless, no downtime | May require multiple treatments for best results |
| Laser Therapy | Effective for fine lines, wrinkles, and pigmentation | Can cause discomfort and require downtime |
| Microneedling | Improves skin texture, reduces scars and wrinkles | Can be uncomfortable, may require multiple sessions |
Choosing between cold plasma, laser, and microneedling depends on your skin needs and what you’re comfortable with. Talking to a skincare expert can help pick the right treatment for you.
FAQs
If you’re thinking about using a cold plasma skin treatment at home, you might have questions. Here are answers to some common questions about the best cold plasma devices for home use.
How often should I use a cold plasma device?
For the best results, use a cold plasma device 2-3 times a week. Being consistent is important. But, don’t overdo it, as it can irritate your skin.
What results can I expect from using a cold plasma device?
Cold plasma devices can help with many skin issues, including:
- Fine lines and wrinkles
- Skin texture and tone
- Acne and blemishes
- Dark spots and hyperpigmentation
Results can vary based on your skin and the device. But, many people see improvements after a few weeks of use.
Are there any side effects or risks associated with cold plasma devices?
Cold plasma devices are usually safe and well-tolerated when used correctly. Some might experience mild side effects, such as:
- Temporary redness
- Skin sensitivity
- Dryness or flaking
If you have sensitive skin or skin conditions, talk to a dermatologist before using a cold plasma device.
Can anyone use a cold plasma device?
Cold plasma devices work for most skin types. But, there are some exceptions. Avoid using them if you:
| Contraindication | Reason |
|---|---|
| Have active acne or open wounds | May cause further irritation or infection |
| Are pregnant or breastfeeding | Safety not established |
| Have a pacemaker or metal implants | May interfere with device function |
| Have a history of skin cancer | Consult with a doctor first |
“Cold plasma devices offer a safe and effective way to achieve professional-level skin care results at home, as long as you choose a high-quality device with advanced safety features and use it as directed.”
Understanding what cold plasma devices can and can’t do helps you decide if they’re right for you.
The Future of Cold Plasma Technology in Home Skincare
The cold plasma skin treatment is getting more popular. It seems the future of this skin rejuvenation technology is bright. Skincare companies and researchers are finding new ways to use cold plasma for anti-aging plasma treatment and more.
One exciting thing about cold plasma tech is the chance for more precise treatments. Scientists are working on devices that can target specific skin areas. This could help reduce fine lines, wrinkles, and other aging signs better.
Another area of research is mixing cold plasma with other skincare tech. For instance, some studies are looking at using cold plasma with light therapy or microneedling. These combos might offer deeper anti-aging benefits and better skin health.
“The future of cold plasma technology in home skincare is incredibly exciting. As research continues to advance, we can expect to see more innovative devices and treatment options that deliver even better results for those looking to maintain youthful, healthy-looking skin.”
As cold plasma tech evolves, we’ll likely see more affordable, easy-to-use devices. This could make cold plasma skin treatment available to more people. It will let more folks enjoy this advanced skin rejuvenation technology from home.
| Future Development | Potential Benefits |
|---|---|
| Targeted treatments | More precise and effective results |
| Combination treatments | Enhanced anti-aging benefits and improved skin health |
| Affordable and user-friendly devices | Increased accessibility for home use |
Conclusion
Cold plasma devices are changing the game for at-home skin care. They can tackle many skin issues, like fine lines and uneven skin tone. These devices boost collagen, making your skin look younger and more radiant.
When picking a cold plasma device, look at its effectiveness, ease of use, safety, and cost. Top picks like the Mirari Cold Plasma Device and the NuDerma Portable Handheld offer advanced tech and easy use. They also show great results.
Adding a cold plasma treatment to your routine can really improve your skin. As this tech gets better, we’ll see even more amazing devices. This means you can get top-notch skin care right at home.
Key Takeaways
- Cold plasma devices offer an effective, non-invasive solution for various skin concerns
- At-home cold plasma treatments provide convenience and accessibility
- Fibroblast plasma therapy stimulates collagen production and improves skin elasticity
- Portable plasma devices are gaining popularity for their ease of use and results
- The top 7 cold plasma devices will be reviewed to help readers make an informed choice
FAQs
What is a cold plasma device, and how does it work?
A cold plasma device is a tool for your skin. It creates a stream of cold plasma that you apply to your skin. This plasma helps your skin by making it look younger and healthier.
The device works by turning a gas, like argon or helium, into plasma. This plasma is safe for your skin.
What are the benefits of using a cold plasma device at home?
Using a cold plasma device at home has many benefits. It can make your skin look smoother and more even-toned. It also helps with acne scars and makes your skin glow.
Home treatments are easy and don’t cost as much as going to a salon.
Is it safe to use a cold plasma device at home?
Yes, it’s safe to use a cold plasma device at home if you follow the instructions. Look for devices with safety features and check if they’re certified. Always be careful and avoid sensitive areas.
How often should I use a cold plasma device for the best results?
The best frequency depends on the device and your skin. Start with 2-3 times a week for a few weeks. Then, use it 1-2 times a week to keep your skin looking good.
Listen to your skin and follow the manufacturer’s advice to find the right frequency for you.
Can cold plasma devices be used on all skin types?
Cold plasma devices work for most skin types, even sensitive skin. But, if you have special skin concerns, talk to a dermatologist first. Some devices have settings for different skin types, so choose the right one for you.
How long does it take to see results from using a cold plasma device?
Results can vary based on your skin and how often you use it. Some people see improvements quickly, while others take longer. Be patient and use it regularly for the best results.
Can I combine cold plasma treatments with other skincare products or procedures?
Yes, you can use cold plasma treatments with other skincare products and procedures. But, be careful and follow the guidelines. Some treatments, like retinoids, might need a break before or after using a cold plasma device.
Related articles
Made in USA