TPO in nail products stands for trimethylbenzoyl diphenylphosphine oxide, a photoinitiator chemical essential for curing gel nail polish under UV or LED light.[1] While the European Union banned TPO on September 1, 2025, due to reproductive toxicity concerns from animal studies, it remains fully legal in the United States, where the FDA applies risk-based regulatory standards.[2] Understanding TPO’s function, safety profile, and alternatives helps consumers and professionals make informed choices about gel manicure products.
Understanding TPO in Nail Products: The Basics
TPO Definition and Chemical Name
Trimethylbenzoyl diphenylphosphine oxide, also known as diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide, is a specialized photoinitiator compound critical to UV-curable and LED-curable gel nail formulations.[1] Identified by CAS number 75980-60-8, this organic molecule contains phosphine oxide functional groups that react specifically to ultraviolet wavelengths between 340-405 nanometers.
The chemical classification places TPO among photoinitiators—compounds that absorb light energy and trigger polymerization reactions. Its purpose extends beyond cosmetics to industrial applications including printing inks, dental materials, and protective coatings requiring rapid photochemical curing.
What TPO Does in Gel Nail Products
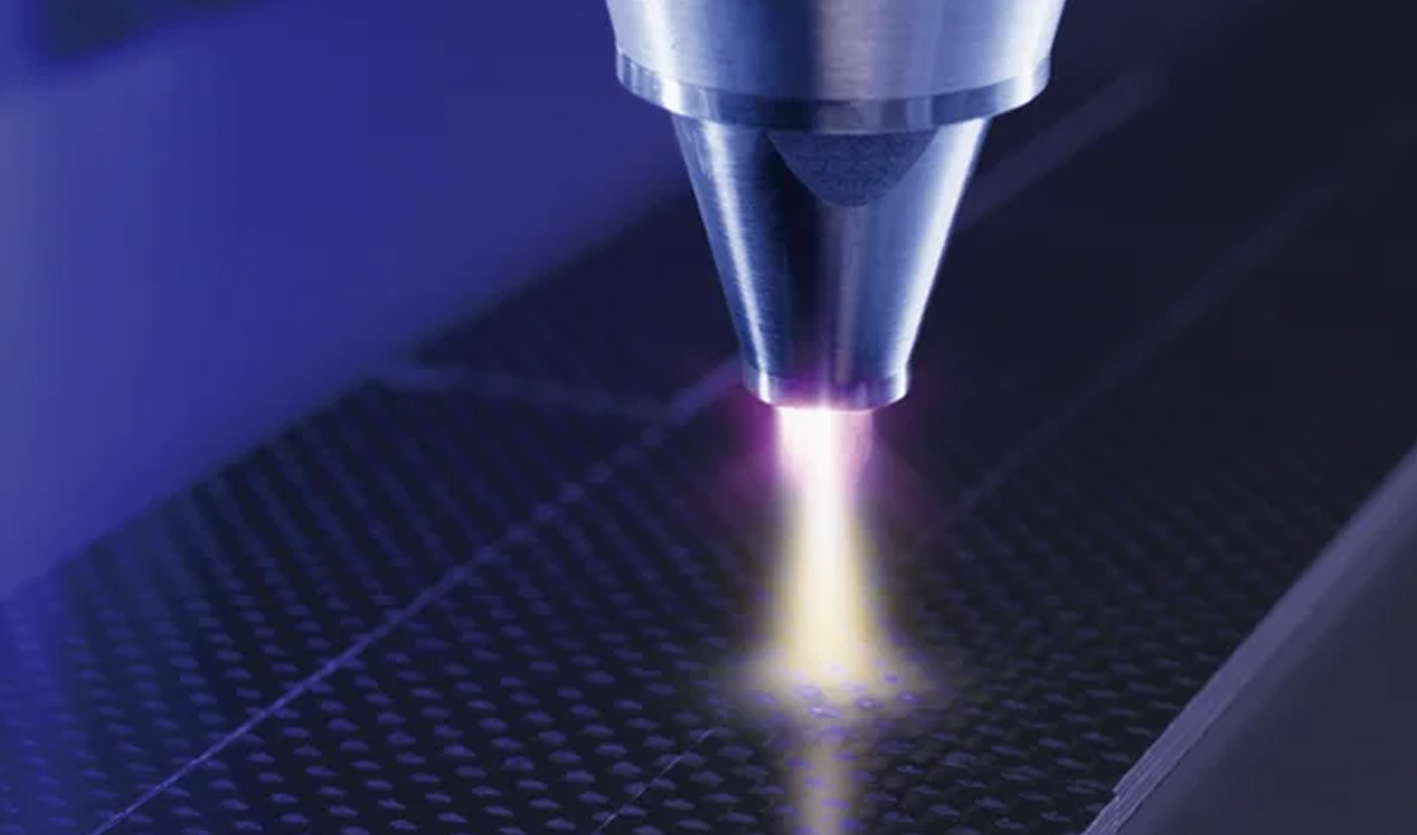
TPO functions as the essential catalyst transforming liquid gel polish into durable, hardened coatings. During UV lamp curing or LED lamp curing, TPO molecules absorb photons and generate free radicals—highly reactive molecular fragments that initiate the polymerization process.[1]
The Polymerization Sequence:
- Light exposure breaks specific chemical bonds within TPO molecules
- Free radicals form and seek bonding opportunities with surrounding compounds
- Monomers and oligomers begin linking into extended polymer chains
- Cross-linking occurs between polymer strands, creating three-dimensional networks
- The liquid gel transforms into a solid, glossy coating adhered to the nail surface
This converting process from liquid gel to solid polymer typically completes in 30-60 seconds under LED lamps, making TPO exceptionally efficient for salon workflows. The deep cure capability ensures complete polymerization throughout thick gel layers, from the nail plate contact point to the glossy outer surface.
Where TPO Is Found
TPO appears exclusively in light-curable nail products requiring UV or LED activation. Common applications include gel polish systems for color application, builder gel formulations for nail extensions, and base coats and top coats that seal and protect manicures.[3]
TPO-Containing Products:
- Traditional gel nail polish (color coats)
- Builder gels for sculptured extensions
- Chrome gels and magnetic cat-eye formulas
- 3D nail art gels for dimensional designs
- Gel extensions and nail enhancements
- Hard gel overlay systems
Products that do NOT contain TPO include regular nail polish (air-dry lacquer), acetone removers, cuticle oils, nail primers, and non-curing topcoats. Understanding this distinction helps consumers identify when TPO exposure may occur.
Similar to how advanced dermatology treatments target specific skin concerns through precise mechanisms, photoinitiators like TPO serve targeted functions in cosmetic chemistry—enabling rapid transformation through controlled photochemical reactions.
How TPO Works: The Science Made Simple
The Curing Process Explained
The UV/LED light absorption mechanism begins when specific wavelengths penetrate the gel polish layer. TPO molecules contain chromophore structures—molecular regions that absorb particular light wavelengths efficiently.[1]
Upon absorbing sufficient photon energy, TPO undergoes homolytic cleavage, splitting into reactive free radical species. These free radicals attack carbon-carbon double bonds in acrylate monomers, creating new radical centers that propagate through the gel formula in chain reactions.
The polymerization process occurs in three distinct phases: initiation (free radical generation), propagation (chain growth), and termination (radical recombination). This step-by-step progression happens within milliseconds once adequate light exposure begins, with the entire curing cycle completing in under one minute for properly formulated gels.
Why Salons Use TPO
Professional nail technicians favor TPO-based formulations for multiple performance advantages that enhance both service efficiency and client satisfaction. Fast curing benefits dramatically reduce appointment times, with complete polymerization occurring in 30-60 seconds under LED lamps compared to 2-3 minutes required by older photoinitiator systems.[1]
TPO Performance Advantages:
| Benefit | Impact | Professional Value |
|---|---|---|
| Deep cure capability | Complete polymerization in thick gel layers | Enables nail extensions and builder gel applications |
| Oxygen inhibition reduction | Minimizes sticky inhibition layer formation | Creates tack-free finish requiring less cleanup |
| High shine finish | Professional glossy appearance without buffing | Eliminates need for additional shine-enhancing steps |
| Long-lasting durability | Maintains wear resistance 2-3 weeks | Reduces client return frequency for maintenance |
| Low yellowing properties | Preserves color accuracy in clear formulas | Critical for French manicures and light pink shades |
The superior performance in professional nail products made TPO the industry standard photoinitiator throughout the 2010s and early 2020s. Its chemical efficiency allows formulators to achieve optimal curing with concentrations of 2-5%, lower than many alternative photoinitiators requiring 6-8% loading.
TPO Performance vs Other Photoinitiators
Curing time optimization represents TPO’s most significant advantage over alternatives like camphorquinone or benzophenone derivatives. Independent laboratory testing demonstrates 40-50% faster cure speeds compared to traditional photoinitiator systems.[3]
Complete polymerization effectiveness varies substantially among photoinitiators. TPO achieves >95% monomer conversion in gel formulations, while some alternatives plateau at 85-90% conversion, leaving residual uncured components that may cause skin sensitization or reduced durability.
Professional performance standards in the nail industry prioritize consistency, reliability, and minimal service failures. TPO’s broad compatibility with both UV (365-380nm) and LED (395-405nm) lamp technologies simplified equipment requirements for salons. Color stability over time shows TPO-cured gels maintain pigment accuracy for 3-4 weeks without significant fading or discoloration, particularly in challenging pastel and neon shades.
TPO Safety: What You Need to Know
The EU Ban Explained (September 2025)
The European Cosmetics Regulation EC1223/2009 provides the framework prohibiting substances classified as carcinogenic, mutagenic, or toxic for reproduction (CMR).[2] Commission Regulation EU 2025/877 specifically added TPO to Annex II—the list of prohibited ingredients—following its reclassification under chemical safety legislation.
The CMR Category 1B substance classification applies to chemicals for which animal evidence strongly suggests presumed human reproductive toxicity. This categorization triggered automatic cosmetic prohibition under EU law, requiring no additional regulatory review or safety assessment.
September 2025 ban date implementation occurred without transition periods or grace intervals. From September 1, 2025, manufacturers cannot place TPO-containing products on the market, retailers cannot sell existing inventory, and salon professionals cannot use TPO products for client services.[2]
The UK ban timeline projects implementation by December 31, 2026, following Brexit-related regulatory independence. The United Kingdom typically harmonizes with EU cosmetic standards but implements changes on delayed schedules to allow industry preparation.
Scientific Evidence Behind Safety Concerns
Animal studies findings centered on reproductive toxicity observed in male rodents exposed to high-dose oral ingestion of TPO.[4] Laboratory rats receiving 100-300 mg/kg body weight daily for extended periods showed testicular abnormalities including reduced sperm production, altered hormone levels, and histological changes in reproductive tissues.
To contextualize these experimental doses: a 70 kg (154 lb) human would need to ingest 7,000-21,000 mg of pure TPO daily to match rodent study exposures—thousands of times higher than any cosmetic exposure scenario.
Study Parameters:
- Oral gavage administration (forced ingestion)
- Daily dosing for weeks or months
- Effects observed only at doses >100 mg/kg
- No topical exposure studies conducted
- No human reproductive harm documented
Reproductive toxicity concerns prompted European Chemicals Agency review in 2021, upgrading TPO from Category 2 (suspected toxicant) to Category 1B (presumed toxicant). The carcinogenic substance classification debate centers on whether hazard potential identified in extreme experimental conditions justifies banning products with negligible real-world exposure.
The toxic for reproduction designation under CLP Regulation (EC) No 1272/2008 applies the precautionary principle—restricting substances with theoretical harm potential regardless of actual human exposure levels or routes.
Real-World Human Exposure Analysis
Topical exposure vs oral ingestion differences represent the critical distinction regulators evaluate. Rodent studies administered TPO through gastrointestinal routes, ensuring systemic absorption and organ distribution. Cosmetic gel polish application involves brief topical contact with minimal dermal absorption.[4]
Human exposure levels in nail salons remain extraordinarily low. Professional nail technicians experience the highest occupational exposure through repeated skin contact during liquid gel application, yet cumulative exposure remains below 500 mg weekly even for full-time professionals performing 30+ gel applications daily.
The Cosmetic Ingredient Review (CIR) assessment concluded that TPO at typical concentration levels (≤5% in gel polish) presents minimal safety concerns when used as intended.[5] The assessment emphasized that proper curing eliminates reactive TPO, transforming it chemically into polymer-bound forms unavailable for skin absorption.
Exposure Reality:
- TPO sealed in hardened gel cannot be absorbed through intact skin
- Gel sits on nail plate composed of dead keratinized cells
- No blood vessels or living tissue in nail plate structure
- Typical concentration levels provide safety margins exceeding 1000-fold
- Post-cure extractable amounts <0.2 parts per million
Minimal risk from nail plate contact reflects anatomical barriers preventing systemic absorption. Unlike skin elsewhere on the body, the nail plate lacks active cellular metabolism, blood supply, or absorption pathways. Any potential exposure occurs only during liquid gel application to surrounding skin—contact lasting 5-10 seconds before curing.
Expert Opinions on Safety
Dermatologist perspectives emphasize evidence-based risk assessment over precautionary restrictions. Dr. Bill Dobos, cosmetic scientist at the University of Cincinnati, noted: “TPO starts that reaction that gets it to cure. When used as intended in gel manicures, exposure is minimal and the ingredient poses extremely low risk.”[4]
Cosmetic chemist assessments highlight the vast differences between hazard identification and actual risk. Kelly Dobos explained: “The doses rats were exposed to in studies were far, far higher than anything one person would be exposed to during a simple manicure.”[4]
Nail industry professional views express frustration with regulations eliminating safe products based on irrelevant toxicology data. Trade associations argue that proper focus should address actual safety concerns like incomplete curing, poor ventilation, or allergenic monomers rather than banning photoinitiators with decades of safe use history.
Risk-based assessment vs hazard-based regulation represents the fundamental philosophical divide. Risk assessment evaluates whether substances pose actual harm under real-world use conditions, while hazard-based approaches ban substances with any theoretical toxicity regardless of exposure probability or magnitude.
Consumer safety standards debate continues within the cosmetic science community, balancing precautionary restrictions against maintaining access to effective, well-tolerated products. No consensus supports TPO prohibition based on current evidence, though consumers retain autonomy choosing TPO-free alternatives if preferred.
While discussions about cosmetic ingredient safety focus on topical exposure, emerging technologies like the Mirari Cold Plasma Device demonstrate how therapeutic applications can deliver controlled biological effects through precise mechanisms—a stark contrast to the incidental, minimal exposures from cosmetic products.
TPO in the United States: Current Status
US Regulatory Position
FDA cosmetics regulation operates under the Federal Food, Drug, and Cosmetic Act, with specific authority over ingredient safety outlined in 21 CFR (Code of Federal Regulations).[6] Currently, no ban or restrictions on TPO exist within US regulatory frameworks, and the FDA has issued no safety warnings or consumer alerts regarding TPO in gel nail products.
The US regulatory status vs EU approach reflects fundamentally different philosophies. American regulation requires demonstrating actual human harm before implementing ingredient bans, whereas European regulation restricts substances with theoretical hazards identified through any toxicological pathway.
Risk-based assessment methodology evaluates ingredients by examining: actual human exposure levels, absorption routes and bioavailability, dose-response relationships, and real-world use patterns. Under this framework, TPO remains acceptable because cosmetic gel manicure exposure doesn’t approach doses causing effects in animal studies.
Why FDA unlikely to ban TPO centers on several factors: three decades of market presence without documented human reproductive harm, massive exposure differences between animal studies and cosmetic use, lack of human epidemiological evidence suggesting risk, and regulatory precedent requiring demonstrated human hazard rather than theoretical concern.[6]
US vs EU Regulatory Differences
Hazard-based regulation under the EU precautionary principle bans substances based on intrinsic properties regardless of exposure magnitude. If animal studies demonstrate potential harm at any dose through any route, substances face restriction.
Risk-based regulation through the US FDA approach considers: whether exposure routes in studies match real-world use, whether doses causing effects reflect actual human exposure, whether protective mechanisms exist (like skin barriers), and whether decades of market use reveal safety signals.
Regulatory Comparison:
| Aspect | European Union | United States |
|---|---|---|
| Philosophy | Hazard-based (precautionary) | Risk-based (evidence-based) |
| Evidence needed | Animal toxicity at any dose | Human harm or high-probability risk |
| Ban timeline | Rapid (months) | Extended review (years) |
| Industry input | Limited | Substantial |
| Consumer choice | Restricted for safety | Maintained with disclosure |
Global market divergence creates complex challenges for international beauty brands. Companies serving both markets must maintain dual inventory systems—TPO-free formulations for European distribution and conventional products for American markets—increasing costs and supply chain complexity.
Consumer Awareness Trends in America
Market research reveals a 65% surge in “TPO-free” searches among US consumers following EU ban announcements in mid-2025.[7] Despite no US regulatory requirement, consumer demand increasingly favors reformulated alternatives.
Surveys indicate 38% of US customers requesting TPO-free options when visiting nail salons, even in states with no local restrictions. This consumer-driven market shift occurs independently of government action, driven by media coverage of European bans and social media discussions amplifying safety concerns.
Market research on consumer preferences shows age-related patterns: younger consumers (18-34) more likely to request TPO-free products (45%), middle-aged consumers (35-54) showing moderate concern (35%), and older consumers (55+) largely unconcerned (22% requesting alternatives).
Voluntary brand reformulation trends accelerate across major manufacturers. Leading brands including OPI, CND, and Essie announced plans for complete TPO elimination by 2026-2027, driven by global market harmonization rather than US regulatory pressure.
Should US Consumers Be Concerned?
Evidence-based safety assessment consistently indicates minimal risk from properly applied and cured gel manicures containing TPO.[5] Expert consensus emphasizes that occasional gel manicure consumers face negligible exposure, particularly when salons follow proper application techniques minimizing skin contact.
When concerns may be warranted include scenarios involving pregnant women preferring precautionary avoidance of any theoretical reproductive exposures, individuals with compromised skin barriers or dermatological conditions, professional nail technicians with daily occupational exposure over decades, and individuals with heightened chemical sensitivity or anxiety regarding ingredient safety.
Personal choice considerations should balance: scientific evidence showing minimal risk at cosmetic exposure levels, individual risk tolerance and precautionary preferences, availability and cost of TPO-free alternatives, and trust in regulatory authorities and expert opinions.
The scientific consensus supports safety for typical consumer use, while acknowledging that personal values and preferences justify choosing alternatives when available. Similar to how patients explore various therapeutic options at Mirari Doctor, consumers deserve access to comprehensive safety information enabling informed personal decisions.
TPO-Free Alternatives and Product Options
Alternative Photoinitiators Overview
TPO-L (ethyl trimethylbenzoyl phenylphosphinate) serves as the primary replacement photoinitiator in reformulated gel polishes.[3] Its structural similarity to TPO provides comparable curing performance while avoiding reproductive toxicity classification under European chemical safety regulations.
BAPO (bis-acylphosphine oxide) represents another high-performance alternative. BAPO demonstrates excellent cure speeds and depth penetration, though raw material costs run approximately 30% higher than TPO, contributing to price increases in reformulated products.
Additional alternatives include phenyl bis(2,4,6-trimethylbenzoyl)-phosphine oxide for specialized applications, hydroxycyclohexyl phenyl ketone offering broad UV absorption, camphorquinone (CQ) primarily used in dental composites but limited by yellowing, Irgacure 819 providing efficient LED compatibility, ITX photoinitiator for rapid surface cure, and benzophenone-1 systems for combination photoinitiator formulations.
TPO-L: The Leading Replacement
Modified molecular structure in TPO-L involves replacing a benzene ring with an ethoxy group.[3] This seemingly minor structural change dramatically alters biological activity and toxicity profiles, explaining why TPO-L avoids reproductive toxicity classification.
The lower toxicity profile stems from reduced reactivity with biological tissues. Animal studies using similar high-dose protocols that flagged TPO show no reproductive effects with TPO-L, supporting its regulatory approval across all major markets.
Comparable curing performance means consumers and professionals notice no difference in application, cure time, or final manicure quality. Independent testing shows cure completion within 5-10% of TPO-based formulations, maintaining professional standards without performance compromise.
EU approval status confirms TPO-L meets current European cosmetic safety standards. Its chemical similarity to TPO allows manufacturers to reformulate without complete formula redesign, minimizing development costs and time-to-market for compliant products.
Popular TPO-Free Brands in the US
Several major brands offer TPO-free gel polish options even absent US regulatory requirements:
Available TPO-Free Brands:
- OPI Intelli-Gel system: Reformulated 2025 for global compliance
- Manucurist Green Flash LED gel polish: European brand with longstanding TPO-free commitment
- Aprés Nail Gel Couleur: Professional line marketed as clean beauty alternative
- Zoya gel polish lines: Emphasizes “safer” ingredient positioning
- Ella+Mila: Vegan, cruelty-free, and TPO-free certification
- PLA Gel Polish: Budget-friendly TPO-free option
- Bio Sculpture: South African brand with early TPO-free reformulation
- GND Canada: TPO-free and HEMA-free combination formulas
Consumer access varies regionally, with TPO-free options more prevalent in major metropolitan areas and coastal markets compared to rural locations with limited salon competition.
Performance Comparison Table
| Metric | TPO-Based | TPO-L Based | BAPO Based | Traditional Polish |
|---|---|---|---|---|
| Curing speed | 30-45 seconds | 30-50 seconds | 25-40 seconds | Air dry 10-15 min |
| Durability | 2-3 weeks | 2-3 weeks | 2-3 weeks | 3-7 days |
| Shine quality | Excellent | Excellent | Excellent | Good |
| Color stability | Very good | Excellent | Excellent | Good |
| Price premium | Baseline | +15-20% | +25-30% | -40% |
| US availability | Widespread | Growing | Limited | Universal |
Wear resistance testing shows negligible differences between TPO and TPO-L formulations, with both maintaining adhesion and preventing chipping for 14-21 days under normal conditions. BAPO-based formulations demonstrate slightly superior long-term color retention, particularly in challenging neon and pastel shades prone to fading.
How to Identify TPO in Products
Reading Product Labels
INCI ingredient list location appears on product packaging, typically on the back panel or bottom label of gel polish bottles.[1] US regulations require ingredient disclosure for consumer products but provide more flexibility for professional-only products marketed exclusively to licensed technicians.
Official chemical names to look for include “Trimethylbenzoyl Diphenylphosphine Oxide,” “Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide,” or the simplified form “TPO” in parenthetical notation.
CAS number 75980-60-8 reference provides definitive identification. Unlike common names that vary across languages and regions, Chemical Abstracts Service (CAS) numbers uniquely identify specific chemical compounds internationally.
Label Reading Tips:
- Ingredients listed in descending concentration order
- Photoinitiators typically appear in middle portion of ingredient lists
- Concentrations of 2-5% place TPO as 5th-10th ingredient typically
- “TPO-free” claims should appear prominently if applicable
- Professional products may lack complete INCI disclosure
Ingredient disclosure requirements differ between consumer retail products (requiring full INCI lists) and professional salon products (often exempt from complete disclosure). This regulatory gap complicates consumer research when products lack clear labeling.
TPO-Free Certification and Claims
Marketing claims verification requires scrutiny, as terms like “clean,” “safe,” or “non-toxic” lack regulatory definitions. Only specific claims like “does not contain TPO” or “TPO-free formulation” provide verifiable information.
Brand disclosure practices vary significantly. Leading manufacturers now prominently advertise TPO-free status, while smaller producers may not proactively disclose photoinitiator choices without direct consumer inquiry.
TPO-free labeling standards remain voluntary in the US market. Unlike allergen labeling in food products, cosmetics face minimal requirements for highlighting ingredient absences, leaving disclosure to manufacturer discretion.
Third-party testing certifications remain limited in cosmetics. Independent verification programs like NSF, Leaping Bunny (cruelty-free), or USDA Organic don’t specifically certify TPO-free status, requiring consumers to trust manufacturer claims.
Questions to Ask at the Salon
Effective communication with nail professionals ensures informed service decisions. Salon product ingredient inquiries should include:
Essential Questions:
- “Are the gel polishes you use TPO-free?”
- “Which specific photoinitiator does your gel system contain?”
- “Can you provide ingredient lists or Safety Data Sheets for your products?”
- “Do you offer both TPO-containing and TPO-free options?”
- “How do you ensure proper curing to minimize any ingredient exposure?”
Professional nail technician education varies widely. While licensed technicians complete state-required training, ingredient chemistry education remains minimal. Many technicians may need to check product labels or contact manufacturers for specific photoinitiator information.
TPO-free options availability depends on salon size, location, and clientele. High-end salons in metropolitan areas more commonly stock reformulated alternatives, while smaller establishments may use existing inventory until depleted.
Proper curing techniques confirmation matters more than specific photoinitiator type for exposure minimization. Asking about lamp wattage, cure times, and skin contact prevention protocols demonstrates informed consumer engagement.
Safe Gel Manicure Practices
Professional Application Standards
Proper gel nail application techniques begin with thorough nail preparation including gentle buffing, cuticle management, and dehydration to ensure optimal adhesion.[8] Skilled technicians apply thin, even coats—typically 0.5-1mm thick—allowing complete light penetration and thorough polymerization.
Avoiding skin contact during application represents the most critical safety practice. Professional application keeps gel polish on the nail plate without flooding cuticles, touching surrounding skin, or pooling along nail edges.
Application Safety Protocol:
- Cleanse nails and hands thoroughly before service
- Apply thin base coat layer, curing for recommended duration
- Apply color coat avoiding cuticle contact, cure completely
- Apply thin top coat for sealing and shine, final cure
- Cleanse any tacky residue with approved cleanser
- If skin contact occurs, immediately remove uncured product before curing
UV lamp curing vs LED lamp curing presents different exposure considerations. UV lamps emit broader wavelength spectrum (340-380nm) requiring 2-3 minute cure times, while LED lamps provide targeted wavelengths (395-405nm) completing polymerization in 30-60 seconds.
Correct curing time for complete polymerization varies by product formulation and lamp wattage. Under-curing leaves reactive monomers and photoinitiators available for skin contact, while proper curing eliminates safety concerns associated with uncured components.
UV/LED Lamp Safety
UV radiation exposure concerns extend beyond chemical ingredient safety to dermatological effects of ultraviolet light itself.[8] While gel curing lamps emit less UV than outdoor sun exposure, repeated exposure accumulates over time, potentially contributing to photoaging and theoretical skin cancer risk.
Skin protection recommendations from dermatologists include applying broad-spectrum sunscreen (SPF 30+) to hands and wrists 20 minutes before salon appointments, using fingerless UV-blocking gloves during curing, reapplying sunscreen if hands are washed immediately before service, and limiting gel manicure frequency to every 3-4 weeks minimum.
Sunscreen application before salon visits provides simple, effective protection. Water-resistant formulations prevent removal during standard hand washing before nail services. LED lamp benefits include dramatically reduced UV output compared to traditional UV lamps, faster cure times minimizing total exposure duration, narrower wavelength emission spectrum, and lower energy consumption with extended bulb lifespan.
Surface curing verification ensures complete polymerization. When the sticky inhibition layer disappears and polish surface becomes tack-free, polymerization is complete and exposure risk eliminated.
Nail Health Maintenance
Preventing damage and brittleness requires understanding that gel removal technique causes more nail harm than gel polish itself.[8] Aggressive scraping, forceful peeling, and excessive filing create the mechanical trauma responsible for nail thinning and brittleness.
Proper Removal Protocol:
- Soak removal wraps or foils for full 10-15 minutes
- Allow acetone sufficient time to dissolve polymer matrix
- Use gentle sliding motion with wooden pusher to remove softened gel
- Never force removal or aggressively scrape
- Buff only surface layer; avoid aggressive filing of nail plate
- Apply cuticle oil and moisturizer after removal
Rest periods between gel manicures allow natural nail recovery. Dermatologists recommend 2-3 week breaks every 8-12 weeks of continuous gel wear to prevent cumulative keratin depletion.
Protective base coat usage creates barrier layers between natural nail and pigmented gel polish. Quality base coats prevent staining and facilitate easier removal while protecting the keratin structure.
Long-term nail care strategies include maintaining adequate hydration through cuticle oil application, consuming biotin-rich foods or supplements supporting keratin health, avoiding nail biting or picking behaviors, wearing protective gloves for cleaning and gardening, and scheduling regular professional manicures with trained technicians.
Consumer Decision-Making Guide
When to Choose TPO-Free Products
Personal risk tolerance assessment guides individual choices absent regulatory mandates or consensus safety warnings. Consumers preferring precautionary approaches may select TPO-free alternatives despite limited evidence suggesting actual human risk from proper gel manicure use.
Pregnancy and reproductive health considerations warrant extra caution. While no evidence demonstrates risk from topical gel polish exposure, pregnant women or couples actively pursuing conception may prefer eliminating any theoretical reproductive exposures during sensitive periods.
Frequency of gel manicure use influences cumulative exposure. Weekly gel manicures create higher lifetime exposure than monthly intervals, though exposure remains minimal even with frequent applications when proper techniques are followed.
Occupational exposure concerns apply primarily to professional nail technicians performing 20-40 gel applications daily. Cumulative occupational exposure over decades may justify precautionary selection of TPO-free products despite absence of documented health effects in nail professional populations.
Cost and Availability Factors
Price premiums for TPO-free formulations typically range 15-25% higher than conventional gel polishes.[7] Reformulation costs, specialized raw materials, and limited production volumes contribute to elevated pricing, though costs should normalize as TPO-free becomes industry standard.
Market Availability by Region (2025):
| Location Type | TPO-Free Availability | Typical Price Premium |
|---|---|---|
| Major metro salons | 70-85% offer options | 15-20% service increase |
| Suburban salons | 50-65% stock alternatives | 20-25% service increase |
| Rural salons | 25-40% carry TPO-free | 25-30% service increase |
| At-home kits | 60-75% of brands | 15-25% retail markup |
Salon adoption rates in major US cities show accelerating transitions. Los Angeles, New York, San Francisco, and Seattle lead adoption with 75-85% of salons offering TPO-free options, while heartland markets show 30-50% availability.
At-home gel kit options increasingly feature TPO-free formulations as major brands reformulate entire product lines rather than maintaining separate conventional and TPO-free SKUs. Online retailers provide broader selection than local beauty supply stores.
Product accessibility and selection continue improving. Major manufacturers project complete transitions to TPO-free formulas by late 2026, driven by global market harmonization and consumer preference trends rather than US regulatory requirements.
Comparing Your Options
Decision Matrix:
| Option | Pros | Cons | Best For |
|---|---|---|---|
| TPO gel polish | Proven performance, lower cost, wide availability | EU banned, consumer concerns growing | Budget-conscious, evidence-focused consumers |
| TPO-free gel | Addresses safety concerns, equivalent performance | Higher cost, limited availability in some areas | Precautionary consumers, pregnant women |
| Traditional polish | Inexpensive, easy removal, no UV exposure | Poor durability (3-7 days), long dry time, chipping | Quick color changes, budget constraints |
| Press-on nails | No chemical exposure, instant application, reusable | Less natural appearance, shorter wear (7-10 days) | Special occasions, travel |
| BYOP strategy | Complete product control, cost savings | Salon may charge application fees, compatibility issues | Highly informed consumers with preferences |
BYOP (bring your own polish) strategies allow complete ingredient control but may incur salon application fees or encounter compatibility issues between consumer products and professional lamp systems.
For Nail Professionals: Industry Impact
Salon Compliance and Inventory
Salon inventory management strategies must address market transitions while managing costs. Recommendations include gradually transitioning to TPO-free products as existing inventory depletes, avoiding large TPO-containing stock purchases given market direction, communicating ingredient changes transparently to established clients, and training staff on TPO-free product application if formulation adjustments needed.
Product transition timeline planning should account for client education requirements, supply chain availability of preferred TPO-free brands, price point adjustments and menu reprinting, and marketing opportunities highlighting safer ingredient positioning.
Client education approaches include displaying ingredient information prominently in service areas, proactively addressing TPO concerns before clients raise questions, explaining US regulatory status versus EU ban rationale, and offering choice between TPO-containing and TPO-free options where possible.
Marketing TPO-free services provides competitive differentiation as consumer awareness grows. Highlighting ingredient transparency, safety consciousness, and commitment to latest formulations attracts health-conscious clientele willing to pay modest premiums.
Business Considerations
Product reformulation impacts extend beyond ingredient changes to supply chain relationships, inventory systems, and pricing structures. Salons must evaluate whether to maintain dual product lines or transition completely to TPO-free formulations.
Cost implications for salons include 15-25% higher product costs, potential service price increases, client communication materials and training, and possible short-term revenue disruption during transition periods.
Consumer demand trends indicate sustained preference shifts toward “cleaner” ingredient profiles regardless of regulatory requirements. Forward-thinking salons position themselves ahead of market trends rather than reacting to regulation.
Competitive positioning opportunities arise from early adoption of TPO-free products, particularly in affluent urban markets with health-conscious clientele. Marketing “ahead of the curve” ingredient safety demonstrates industry leadership.
Industry Best Practices
Client safety considerations should prioritize proper application technique, adequate curing, minimal skin contact, and transparent ingredient communication over specific photoinitiator selection.
Transparent ingredient communication builds trust and demonstrates professionalism. Maintaining product Safety Data Sheets accessible for client review, answering ingredient questions knowledgeably, and avoiding defensive reactions to safety concerns creates positive client relationships.
Continuing education on alternatives keeps professionals informed about evolving formulations, application adjustments, and performance characteristics of TPO-free systems. Manufacturer training programs and industry seminars provide valuable updated information.
Professional certifications from organizations like CND, OPI, or independent nail technology associations enhance credibility and demonstrate commitment to ongoing education and safety standards.
The Future of Nail Product Formulations
Innovation Trends
Safer photoinitiator development continues beyond simple TPO replacement. Research focuses on compounds with improved safety profiles, reduced sensitization potential, faster cure speeds, and broader lamp compatibility.
Bio-sourced formula innovations explore plant-derived photoinitiator precursors and sustainable raw material sourcing. While still in development stages with limited commercial availability, bio-based alternatives represent long-term industry direction.
Improved performance formulations target extending wear time beyond current 2-3 week standards, enhancing color vibrancy and accuracy, simplifying removal processes, and reducing application layer requirements.
Reduced sensitization potential addresses allergic contact dermatitis concerns beyond TPO. Eliminating HEMA, minimizing methacrylates, and developing hypoallergenic formulations create products suitable for chemically sensitive individuals.
Global Regulatory Outlook
Potential US regulatory changes remain unlikely short-term but possible long-term as consumer advocacy groups pressure FDA to reevaluate cosmetic ingredients.[6] The agency’s risk-based philosophy typically requires evidence of actual human harm before implementing ingredient bans.
FDA monitoring of EU developments suggests awareness of international regulatory trends, though American agencies rarely adopt European precautionary approaches without independent safety reviews.
International cosmetic standards convergence faces challenges from divergent regulatory philosophies. US-EU harmonization seems unlikely while fundamental regulatory approaches remain opposed.
Other countries following EU precedent include Switzerland and Norway (through EEA agreements), Morocco (implementing restrictions September 2025), and potentially UK (projected December 2026 ban). Canada evaluates TPO under existing cosmetic safety frameworks.
Market Predictions for 2026-2027
Complete industry transition projections suggest 80-90% of gel polish formulations will be TPO-free by late 2026, driven by voluntary manufacturer reformulation rather than US regulatory mandate.[7]
Consumer preference evolution indicates sustained demand for “cleaner” ingredient profiles regardless of scientific evidence supporting safety. Marketing advantages of TPO-free positioning incentivize complete transitions even in unregulated markets.
Price normalization expectations suggest TPO-free premiums will decrease from current 15-25% to 5-10% as production volumes increase and reformulation becomes industry standard.
Technology advancements in curing systems may reduce photoinitiator requirements entirely through improved lamp efficiency, wavelength optimization, or novel curing mechanisms beyond traditional UV/LED photopolymerization.
Key Takeaways
- TPO remains fully legal and widely used in US nail salons with no FDA restrictions or safety warnings
- EU ban reflects precautionary regulation based on animal studies using extreme oral doses irrelevant to topical cosmetic exposure
- Properly cured gel polish contains negligible extractable TPO (<0.2 ppm) presenting minimal human exposure risk
- TPO-free alternatives like TPO-L deliver equivalent curing performance, durability, and shine at 15-25% higher cost
- 38% of US consumers now request TPO-free options despite no regulatory requirement, driving voluntary reformulation
- Personal choice should balance scientific evidence of minimal risk with individual risk tolerance and precautionary preferences
- Professional nail technicians have higher occupational exposure than occasional consumers but still well below concerning levels
Frequently Asked Questions (FAQs)
What is TPO and why is it in gel nail polish?
TPO (trimethylbenzoyl diphenylphosphine oxide) is a photoinitiator that absorbs UV/LED light energy and triggers the polymerization reaction converting liquid gel polish into solid, durable coatings. It became the industry standard due to fast 30-60 second cure times, deep cure capability, high shine finish, and superior performance compared to alternative photoinitiators.
Is TPO dangerous or toxic?
Current scientific evidence indicates TPO poses minimal health risks when used in gel manicures as intended. The EU ban stems from animal studies involving oral doses thousands of times higher than cosmetic exposure. Expert consensus supports safety for typical consumer use, as properly cured gel polish contains negligible extractable TPO (<0.2 ppm).
Why did Europe ban TPO but the US didn’t?
The EU applies hazard-based regulation under the precautionary principle, banning substances with any animal toxicity evidence regardless of exposure levels. The US FDA uses risk-based assessment, evaluating whether actual human exposure scenarios pose harm. Since real-world gel manicure exposure differs vastly from animal study conditions, the FDA hasn’t restricted TPO.
Can I still get gel manicures with TPO safely?
Yes, occasional gel manicures with TPO-containing products present minimal risk when proper application techniques minimize skin contact and adequate curing ensures complete polymerization. Pregnant women or those with specific reproductive health concerns may prefer TPO-free alternatives as a precautionary measure. Professional nail technicians with daily occupational exposure may also consider TPO-free options.
How can I tell if my gel polish contains TPO?
Check the INCI ingredient list on product packaging for “Trimethylbenzoyl Diphenylphosphine Oxide,” “Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide,” or CAS number 75980-60-8. Consumer gel polish kits must list ingredients, though professional salon products may lack complete disclosure. Contact manufacturers or ask your nail technician for definitive photoinitiator information.
What are TPO-free alternatives and are they as good?
TPO-L (ethyl trimethylbenzoyl phenylphosphinate) serves as the primary replacement, offering equivalent curing performance with modified molecular structure reducing toxicity. Other alternatives include BAPO and various combination photoinitiator systems. Independent testing shows cure times within 5-10% of TPO formulations, with some alternatives offering improved long-term color stability. Performance differences are negligible for consumers.
Should pregnant women avoid TPO in nail products?
While no human studies demonstrate reproductive harm from topical gel polish use, pregnant women may choose TPO-free products as a precautionary measure. Animal studies showing fertility effects involved oral doses vastly exceeding any cosmetic exposure. Occasional gel manicures likely pose negligible risk, but individual comfort levels and risk tolerance should guide personal decisions during pregnancy.
Will TPO be banned in the United States?
No immediate US ban is expected. The FDA’s risk-based regulatory approach requires evidence of actual human harm before implementing ingredient restrictions. TPO’s three-decade use history without documented human reproductive harm makes near-term restrictions unlikely absent new scientific evidence. However, voluntary industry reformulation will make TPO-free products increasingly standard by 2026-2027.
Does TPO affect nail technicians differently than clients?
Nail technicians experience higher cumulative occupational exposure through repeated skin contact during daily gel applications over years or decades. However, even full-time professionals performing 30+ gel services daily remain well below exposure levels causing effects in animal studies. Professional protective measures include minimizing skin contact, ensuring proper ventilation, and using nitrile gloves when handling uncured products.
How much more do TPO-free gel polishes cost?
TPO-free formulations typically carry 15-25% price premiums compared to conventional gel polishes, translating to $3-8 more per salon service or $5-12 more per at-home kit. Reformulation costs, specialized raw materials, and limited production volumes contribute to elevated pricing. Costs should normalize to 5-10% premiums by 2026-2027 as TPO-free becomes industry standard.
References
- European Commission. (2025). TPO in Nail Products – Questions & Answers. https://single-market-economy.ec.europa.eu/sectors/cosmetics/tpo-nail-products-questions-answers_en
- Health Products Regulatory Authority. (2025). TPO added to European Union list of prohibited ingredients. https://www.hpra.ie/news-events/news/article/tpo-added-to-european-union-list-of-prohibited-ingredients
- Cosmetics Business. (2025). European Union bans popular gel nail polish ingredient TPO. https://cosmeticsbusiness.com/european-union-bans-popular-ingredient-tpo
- CNN Health. (2025). Europe bans chemical TPO used in some gel nail polishes, raising safety questions. https://www.cnn.com/2025/09/04/health/gel-nail-polish-chemical-ban-europe-wellness
- Cosmetic Ingredient Review. (2024). Safety Assessment of Photoinitiators in Cosmetics. https://www.cir-safety.org
- U.S. Food and Drug Administration. (2025). Cosmetics Laws & Regulations. https://www.fda.gov/cosmetics/cosmetics-laws-regulations
- Professional Beauty Association. (2025). Consumer Trends in Nail Product Preferences. https://www.probeauty.org
- American Academy of Dermatology. (2025). Gel Manicures: Tips for Healthy Nails. https://www.aad.org/public/everyday-care/nail-care-secrets
Related articles
Made in USA