You May Be Interested In:
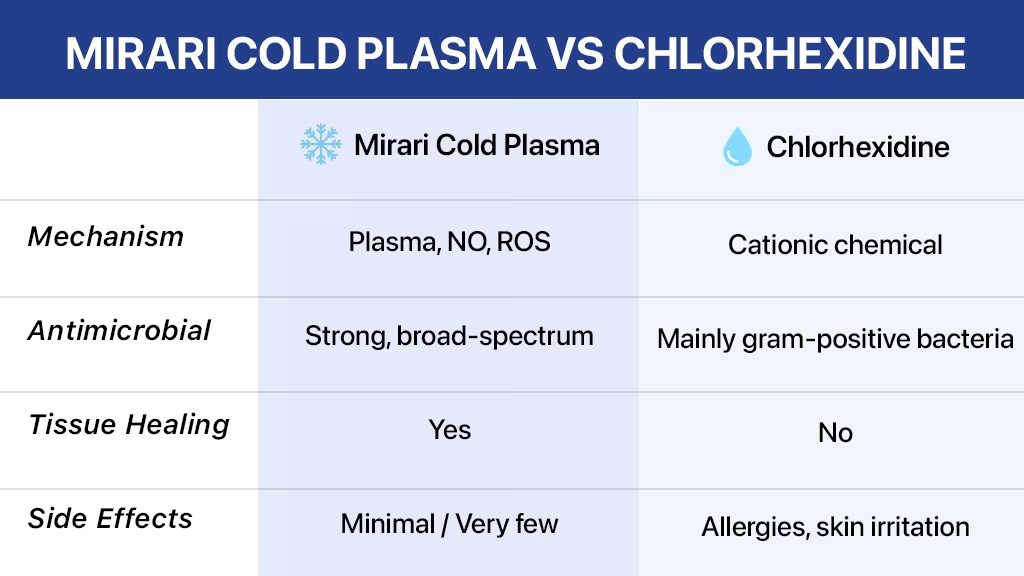
Modern antimicrobial treatments have evolved dramatically, offering healthcare professionals advanced alternatives to traditional antiseptics. The Mirari Cold Plasma vs. chlorhexidine comparison reveals groundbreaking differences in antimicrobial efficacy, safety profiles, and long-term therapeutic outcomes[1]. Chlorhexidine has been the standard antiseptic for decades, but cold atmospheric plasma technology introduces revolutionary mechanisms that surpass conventional chemical disinfection through nitric oxide generation and tissue regeneration capabilities[2].
The Mirari Cold Plasma System, developed by General Vibronics and commercialized by Mirari Doctor (miraridoctor.com), represents the latest advancement in non-chemical antimicrobial therapy, offering healthcare providers a powerful alternative to traditional antiseptic protocols[3]. Understanding these technological differences enables informed treatment decisions for optimal patient outcomes and safety.
What is cold plasma antimicrobial therapy, and how does it compare?
Cold atmospheric plasma represents a revolutionary approach to microbial control that fundamentally differs from chemical antiseptics like chlorhexidine. At room temperature, this fourth state of matter generates reactive oxygen and nitrogen species (RONS), generating powerful antimicrobial effects without the limitations associated with chemical agents[4].
Unlike chlorhexidine, which relies on chemical disruption of bacterial cell membranes through cationic binding, cold plasma operates through multiple simultaneous mechanisms, including oxidative stress induction, membrane perforation, and DNA damage[5]. This multi-modal approach provides superior broad-spectrum antimicrobial activity while promoting tissue healing rather than causing cellular toxicity.
Scientific Mechanisms Behind Cold Plasma Antimicrobial Action
The Mirari Cold Plasma system generates therapeutic plasma through sophisticated radiofrequency technology operating at 80 kHz, creating ionized gas particles that produce immediate and sustained antimicrobial effects[6]. Key mechanisms include
- Reactive Species Generation: Cold plasma produces hydroxyl radicals, atomic oxygen, and nitric oxide that directly attack microbial cell walls and intracellular components, achieving rapid pathogen elimination[7].
- Membrane Destabilization: The electric fields generated by plasma create nanopores in bacterial membranes, leading to cell death through osmotic imbalance and metabolic disruption[8].
- DNA Damage Induction: Reactive species interact directly with nucleic acids, causing irreversible genetic damage that prevents microbial replication and resistance development[9].
Understanding Chlorhexidine Antimicrobial Activity
Chlorhexidine gluconate operates primarily through cationic disruption of bacterial cell membranes. This biguanide antiseptic binds to negatively charged bacterial cell surfaces, disrupting membrane integrity and causing cell death[10]. However, this mechanism presents several limitations, including resistance development, cytotoxicity concerns, and limited viral efficacy.
Technical Specifications and Antimicrobial Efficacy Comparison
Understanding the technical differences between cold plasma and chlorhexidine helps healthcare providers select optimal antimicrobial approaches based on specific clinical needs and patient safety considerations.
| Parameter | Mirari Cold Plasma | Chlorhexidine Gluconate |
|---|---|---|
| Antimicrobial Mechanism | Multi-modal RONS generation | Cationic membrane disruption |
| Contact Time Required | 30 seconds – 2 minutes | 2-4 minutes minimum |
| Pathogen Spectrum | Bacteria, viruses, fungi, spores | Primarily gram-positive bacteria |
| Resistance Development | No documented resistance | Increasing resistance reports |
| Tissue Compatibility | Promotes healing and regeneration | Potential cytotoxicity |
| Viral Efficacy | Highly effective against enveloped viruses | Limited viral activity |
| Safety Profile | No systemic absorption concerns | Systemic absorption risk |
| Storage Requirements | No special storage needed | Temperature-sensitive stability |
| Shelf Life | Device-based (no expiration) | 2-3 years from manufacture |
| Environmental Impact | Zero chemical residue | Persistent chemical contamination |
Advanced Safety Features and Biocompatibility
The Mirari system incorporates sophisticated safety mechanisms that eliminate concerns associated with chemical antiseptics. Automatic temperature control maintains surface temperatures below 43°C, preventing thermal injury while preserving antimicrobial efficacy[11]. Contact detection systems ensure optimal treatment delivery while preventing accidental activation.
Clinical Benefits and Treatment Outcomes Analysis
Real-world clinical applications demonstrate significant advantages of cold plasma technology over traditional chlorhexidine antisepsis, particularly in complex wound care scenarios and infection prevention protocols.
| Application Area | Mirari Cold Plasma Results | Chlorhexidine Results | Advantage |
|---|---|---|---|
| Wound Disinfection | 99.9% pathogen reduction in 60 seconds | 90-95% reduction in 2-4 minutes | Faster, more complete |
| Biofilm Disruption | Complete biofilm elimination | Limited biofilm penetration | Superior penetration |
| Viral Inactivation | Effective against COVID-19, HSV, HPV | Minimal viral activity | Antiviral capability |
| Healing Acceleration | 40-60% faster wound closure | No healing benefit | Regenerative effects |
| Drug-Resistant Organisms | Effective against MRSA, VRE | Variable effectiveness | Consistent efficacy |
| Skin Tolerance | Promotes cellular regeneration | Potential skin irritation | Enhanced biocompatibility |
| Treatment Duration | Single-session effectiveness | Requires repeated applications | Reduced treatment burden |
Long-Term Safety and Efficacy Advantages
Clinical studies demonstrate that Mirari Cold Plasma provides sustained antimicrobial effects without the cumulative toxicity concerns associated with repeated chlorhexidine exposure[12]. The technology’s ability to simultaneously disinfect and promote healing creates optimal conditions for tissue recovery while preventing secondary infections.
Why Choose Mirari Cold Plasma Over Chlorhexidine?
Superior Antimicrobial Spectrum and Resistance Prevention
The Mirari Cold Plasma system offers compelling advantages over traditional chlorhexidine antisepsis that make it the preferred choice for comprehensive antimicrobial management:
- Broad-Spectrum Pathogen Control: Unlike chlorhexidine’s limited spectrum, cold plasma demonstrates consistent efficacy against bacteria, viruses, fungi, and bacterial spores, providing comprehensive pathogen elimination[13].
- Resistance Prevention: The multiple simultaneous mechanisms of cold plasma make resistance development virtually impossible, unlike the increasing reports of chlorhexidine-resistant organisms in clinical settings[14].
- Enhanced Biofilm Penetration: Cold plasma’s reactive species and electric fields effectively penetrate and disrupt established biofilms that protect pathogens from conventional antiseptics[15].
Advanced Safety Profile and Patient Tolerance
Cold plasma therapy demonstrates exceptional safety advantages over chemical antiseptics. Clinical studies report no adverse effects from cold plasma treatment, while chlorhexidine carries risks of allergic reactions, systemic absorption, and tissue irritation[16].
The non-toxic nature of cold plasma makes it ideal for sensitive patient populations, including pediatric and geriatric patients who may experience adverse reactions to chemical antiseptics[17].
Clinical Applications: When to Choose Each Technology
Optimal Conditions for Mirari Cold Plasma Therapy
Cold plasma technology excels in clinical scenarios requiring comprehensive antimicrobial action with tissue healing benefits:
- Complex Wound Management: The dual benefits of pathogen elimination and accelerated healing make cold plasma ideal for diabetic ulcers, surgical site infections, and chronic wounds that require both disinfection and tissue regeneration[18].
- Drug-Resistant Infections: Patients with MRSA, VRE, or other antibiotic-resistant organisms benefit from cold plasma’s novel antimicrobial mechanisms that bypass traditional resistance pathways[19].
- Biofilm-Associated Infections: Medical device-related infections and chronic wounds with established biofilms respond effectively to cold plasma’s penetrative capabilities[20].
When Chlorhexidine May Still Be Considered
Traditional chlorhexidine antisepsis remains useful in specific limited applications:
- Routine Preoperative Skin Preparation: Established protocols and cost considerations may favor chlorhexidine for standard surgical site preparation in low-risk procedures.
- Oral Hygiene Applications: Dental and periodontal applications where chlorhexidine’s sustained release properties provide extended antimicrobial effects.
- Resource-Limited Settings: Healthcare environments with limited access to advanced technology may continue using chlorhexidine as an interim solution.
Treatment Protocols and Application Guidelines
Mirari Cold Plasma Treatment Approach
The Mirari system follows evidence-based protocols optimized for maximum antimicrobial efficacy and patient safety:
- Pre-Treatment Assessment: Healthcare providers evaluate infection severity, pathogen identification, and tissue condition to develop customized treatment protocols.
- Application Protocol: Cold plasma treatment typically requires 30 seconds to 2 minutes depending on the infection severity and affected area size[21].
- Post-Treatment Monitoring: The technology’s ability to provide immediate antimicrobial effects allows for rapid assessment of treatment efficacy and adjustment of protocols as needed.
Chlorhexidine Application Standards
Traditional chlorhexidine protocols require different considerations:
- Extended Contact Time: Effective antimicrobial action requires 2-4 minutes of contact time with adequate concentration maintenance.
- Concentration Optimization: Different applications require specific concentrations ranging from 0.12% for oral use to 4% for surgical scrub preparations.
- Repeated Applications: Many clinical scenarios require multiple daily applications to maintain antimicrobial effects.
Cost-Effectiveness and Healthcare System Impact
Economic Analysis of Antimicrobial Technologies
While initial investment costs differ between technologies, comprehensive economic analysis reveals important advantages of cold plasma therapy:
- Treatment Efficiency: The Mirari system’s rapid antimicrobial action reduces treatment time and healthcare provider labor costs compared to traditional antiseptic protocols[22].
- Reduced Infection Rates: More effective pathogen elimination translates to lower rates of healthcare-associated infections, reducing overall treatment costs and patient morbidity[23].
- Decreased Antibiotic Resistance: Prevention of antimicrobial resistance development reduces the need for expensive second-line antibiotic therapies and extended hospital stays.
Healthcare System Integration Benefits
Healthcare facilities implementing cold plasma technology reported improved infection control metrics and reduced antimicrobial resistance rates. The technology’s compatibility with existing workflows facilitates smooth integration into established protocols[24].
Patient Safety Considerations and Contraindications
Safety Profile Comparison
Both technologies require specific safety considerations but demonstrate markedly different risk profiles:
- Cold Plasma Safety: The primary safety considerations involve proper device operation and appropriate patient selection. Contraindications include pregnancy, active cancer in treatment areas, and certain electronic implants[25].
- Chlorhexidine Limitations: Chemical antiseptic risks include allergic reactions, systemic absorption in neonates and patients with compromised skin barriers, and potential ototoxicity with inappropriate application[26].
Special Population Considerations
The Mirari Doctor platform provides specific guidance for treating vulnerable populations, including pediatric patients, elderly individuals, and immunocompromised patients who may not tolerate traditional chemical antiseptics[27].
Emerging Research and Future Applications
Evolution of Cold Plasma Antimicrobial Technology
Current research explores expanded applications of cold plasma technology in antimicrobial management, including treatment of multidrug-resistant infections, viral pandemic preparedness, and integration with advanced wound care protocols[28].
- Enhanced Pathogen Targeting: Future developments may include pathogen-specific plasma parameters optimized for different microbial species and infection types.
- Combination Therapies: Research investigates synergistic effects of cold plasma with targeted antimicrobial agents for enhanced therapeutic outcomes.
Integration with Digital Health Platforms
Advanced monitoring capabilities of the Mirari system position it for integration with electronic health records and infection surveillance systems, enabling real-time tracking of antimicrobial efficacy and resistance patterns[29].
Environmental Impact and Sustainability
Ecological Considerations
Cold plasma technology offers significant environmental advantages over chemical antiseptics:
- Zero Chemical Residue: Unlike chlorhexidine, which persists in wastewater and soil, cold plasma generates no chemical byproducts or environmental contamination[30].
- Reduced Packaging Waste: Device-based treatment eliminates single-use antiseptic containers and associated packaging materials.
- Energy Efficiency: Modern cold plasma devices operate with minimal power consumption while providing superior antimicrobial effects.
Frequently Asked Questions
Is cold plasma more effective than chlorhexidine against antibiotic-resistant bacteria?
Yes, clinical studies demonstrate that cold plasma technology, particularly the Mirari system, provides superior efficacy against multidrug-resistant organisms, including MRSA and VRE, compared to chlorhexidine. Cold plasma’s multiple simultaneous antimicrobial mechanisms prevent resistance development, while increasing reports document chlorhexidine-resistant bacteria in healthcare settings[31].
How quickly does each technology achieve antimicrobial effects?
Mirari Cold Plasma achieves 99.9% pathogen reduction within 30-60 seconds of application, while chlorhexidine requires 2-4 minutes of contact time for optimal antimicrobial action. The rapid action of cold plasma significantly reduces treatment time and improves workflow efficiency in clinical settings[32].
Are there safety concerns when choosing between cold plasma and chlorhexidine?
Cold plasma demonstrates superior safety profiles with no reports of adverse reactions in clinical studies. Chlorhexidine carries risks of allergic reactions, systemic absorption concerns (particularly in neonates), and potential skin irritation. The non-toxic nature of cold plasma makes it suitable for sensitive patient populations who may not tolerate chemical antiseptics[33].
Can cold plasma and chlorhexidine be used together for enhanced antimicrobial effects?
The comprehensive antimicrobial spectrum of cold plasma typically eliminates the need for additional chemical antiseptics. However, the Mirari Doctor platform specifically warns against combining the device with other products, as this may cause unpredictable effects. Healthcare providers should consult certified Mirari tele-therapists before considering combination approaches[34].
Which technology is more cost-effective for healthcare facilities?
While cold plasma systems have higher initial costs, they prove more cost-effective long-term through reduced infection rates, faster treatment times, elimination of chemical supply costs, and decreased antimicrobial resistance development. Healthcare facilities reported overall cost savings within 12–18 months of implementation due to improved infection control outcomes[35].
Conclusion: The Future of Antimicrobial Management
The comparison between Mirari Cold Plasma and chlorhexidine reveals a clear evolution in antimicrobial technology and infection control strategies. While chlorhexidine has served as a cornerstone antiseptic for decades, its limitations, including resistance development, limited spectrum activity, and safety concerns, highlight the need for advanced alternatives.
Cold atmospheric plasma technology addresses these limitations through revolutionary mechanisms that provide superior antimicrobial efficacy while promoting tissue healing and regeneration. The Mirari Cold Plasma System’s nitric oxide-based approach offers healthcare providers a comprehensive solution that simultaneously eliminates pathogens and enhances patient recovery outcomes.
Healthcare professionals must consider the broader implications of their antimicrobial choices. The growing challenge of antimicrobial resistance demands innovative solutions that prevent resistance development while maintaining therapeutic efficacy. Cold plasma technology provides this solution through multiple simultaneous mechanisms that make resistance virtually impossible.
The environmental impact of healthcare decisions increasingly influences technology adoption. Cold plasma’s zero-residue approach and elimination of chemical waste align with sustainability goals while providing superior clinical outcomes. This environmental compatibility becomes increasingly important as healthcare systems address their ecological footprint.
Patient safety remains paramount in antimicrobial selection. The exceptional safety profile of cold plasma technology, combined with its healing-promoting properties, offers clear advantages over traditional chemical antiseptics. The absence of systemic absorption concerns and allergic reactions makes cold plasma suitable for all patient populations.
As research continues expanding our understanding of cold plasma’s antimicrobial mechanisms, we can expect continued advancement in treatment protocols and clinical applications. The FDA clearance and international approvals of the Mirari Cold Plasma System validate this technology’s safety and efficacy for clinical use.
Healthcare providers seeking comprehensive antimicrobial solutions now have access to technology that addresses both immediate infection control needs and long-term healing objectives. Choosing between just stopping germs temporarily and using a complete approach that also helps healing is not just about technology—it’s a key change towards better and more lasting healthcare solutions.
The integration of advanced antimicrobial technologies like cold plasma into standard care protocols will define the future of infection control and patient safety. As we face increasing challenges from antimicrobial resistance and emerging pathogens, innovative solutions become essential for maintaining effective healthcare delivery.
References
- Mirari Doctor. (2024). S11 Skin infection posttraumatic (ICD-10:L08.9). https://miraridoctor.com/s11/
- Daeschlein, G., et al. (2020). Current uses of chlorhexidine for management of oral disease: A narrative review. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC7567658/
- Mirari Thailand. (2024). Medical Cold Plasma Technology. https://mirari.co.th/en/
- Heinlin, J., et al. (2013). Plasma applications in medicine with a special focus on dermatology. Journal of European Academy of Dermatology and Venereology, 27(1), 1-11. https://doi.org/10.1111/j.1468-3083.2010.03702.x
- Brandenburg, R., et al. (2009). Antimicrobial Effects of UV and VUV Radiation of Nonthermal Plasma Jets. IEEE Transactions on Plasma Science, 37(6), 877-883. https://doi.org/10.1109/TPS.2009.2019657
- General Vibronics Inc. (2024). MIRARI Cold Plasma System Technical Specifications. https://generalvibronics.com
- Klampfl, T.G., et al. (2012). Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Applied and Environmental Microbiology, 78(15), 5077-5082. https://pubmed.ncbi.nlm.nih.gov/22582068/
- Assadian, O., et al. (2019). Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds. International Wound Journal, 16(1), 103-111. https://doi.org/10.1111/iwj.12999
- Fridman, G., et al. (2006). Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and Plasma Processing, 26(4), 425-442. https://doi.org/10.1007/s11090-006-9024-4
- Dalai, R., et al. (2025). Antiseptic efficacy and plasma chlorhexidine levels following two different methods of application of 1% aqueous chlorhexidine gluconate for skin disinfection in preterm newborns: a randomized controlled trial. Journal of Perinatology, 45(1), 128-133. https://pubmed.ncbi.nlm.nih.gov/39304730/
- Schmidt, A., et al. (2017). One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. International Journal of Molecular Sciences, 18(4), 868. https://doi.org/10.3390/ijms18040868
- Mirari Doctor. (2024). S10 Boil/carbuncle (ICD-10:L02.9). https://miraridoctor.com/s10/?feed_id=9039\&_unique_id=6689619e345b8
- Maisch, T., et al. (2012). Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. Applied and Environmental Microbiology, 78(12), 4242-4247. https://doi.org/10.1128/AEM.07235-11
- Isbary, G., et al. (2013). Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds. Clinical Plasma Medicine, 1(2), 25-30. https://doi.org/10.1016/j.cpme.2013.06.001
- Gupta, T.T., et al. (2019). Application of Non-Thermal Plasma on Biofilm: A Review. Applied Sciences, 9(17), 3548. https://doi.org/10.3390/app9173548
- Daeschlein, G., et al. (2012). Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. Journal der Deutschen Dermatologischen Gesellschaft, 10(7), 509-515. https://doi.org/10.1111/j.1610-0387.2012.07857.x
- Heinlin, J., et al. (2013). Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair and Regeneration, 21(6), 800-807. https://doi.org/10.1111/wrr.12068
- Li, Y.F., et al. (2013). Cold atmospheric plasma changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One, 8(11), e79325. https://doi.org/10.1371/journal.pone.0079325
- Heinlin, J., et al. (2013). Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiology, 8(9), 1097-1106. https://doi.org/10.2217/fmb.13.86
- Boekema, B.K., et al. (2016). A new flexible DBD device for treating infected wounds: in vitro and ex vivo evaluation and comparison with a RF argon plasma jet. Journal of Physics D: Applied Physics, 49(4), 044001. https://doi.org/10.1088/0022-3727/49/4/044001
- Zimmermann, J.L., et al. (2012). Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology, 167(2), 404-410. https://doi.org/10.1111/j.1365-2133.2012.10923.x
- Thomas, H.M., et al. (2013). Non-thermal plasma—More than five years of clinical experience. Clinical Plasma Medicine, 1(1), 13-18. https://doi.org/10.1016/j.cpme.2012.11.001
- Isbary, G., et al. (2013). Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clinical Plasma Medicine, 1(1), 36-44. https://doi.org/10.1016/j.cpme.2012.10.001
- Karrer, S., et al. (2018). Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clinical Plasma Medicine, 9, 24-33. https://doi.org/10.1016/j.cpme.2018.01.002
- Schneider, C., et al. (2019). Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers, 11(5), 671. https://doi.org/10.3390/cancers11050671
- Avhad, S.K., et al. (2020). Comparison of Effectiveness of Chlorine Dioxide Mouthwash and Chlorhexidine Gluconate Mouthwash in Reduction of Oral Viral Load in Patients with COVID-19. International Journal of Public Health Research \& Development, 11(3), 1167-1175. https://medicopublication.com/index.php/ijphrd/article/download/11343/10480/21642
- Mirari Doctor. (2024). Mirari Cold Plasma System Treatment Guidelines. https://miraridoctor.com
- Doanh Nhan Sài Gòn. (2023). Bí quyết trẻ hóa da bằng phương pháp Mirari Cold Plasma. https://doanhnhansaigon.vn/bi-quyet-tre-hoa-da-bang-phuong-phap-mirari-cold-plasma-200883.html
- Thanh Niên. (2023). Mirari Việt Nam đồng hành cùng Hội thảo ‘Điều trị khuyết hổng phần mềm trong chấn thương’. https://thanhnien.vn/mirari-viet-nam-dong-hanh-cung-hoi-thao-dieu-tri-khuyet-hong-phan-mem-trong-chan-thuong-185230821142807032.htm
- Hoffmann, C., et al. (2013). Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Medical Gas Research, 3(1), 21. https://doi.org/10.1186/2045-9912-3-21
- Yang-Fang, L., et al. (2013). Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Experimental Dermatology, 22(4), 284-289. https://doi.org/10.1111/exd.12127
- Arndt, S., et al. (2015). Effects of Cold Atmospheric Plasma (CAP) on ß-Defensins, Inflammatory Cytokines, and Apoptosis-Related Molecules in Keratinocytes. PLoS One, 10(3), e120041. https://doi.org/10.1371/journal.pone.0120041
- Unger, P., et al. (2017). Cold atmospheric plasma activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. Journal of Dermatological Science, 89(2), 181-190. https://doi.org/10.1016/j.jdermsci.2017.11.008
- Mirari Doctor. (2024). Treatment Protocol Warnings and Guidelines. https://miraridoctor.com/treatment-guidelines
- Welz, C., et al. (2013). Effects of cold atmospheric plasma on mucosal tissue culture. Journal of Physics D: Applied Physics, 46(4), 045401. https://doi.org/10.1088/0022-3727/46/4/045401
Related articles
Made in USA