FDA approvals for cold plasma devices represent a significant milestone in modern medical technology, marking the transition from experimental treatments to clinically validated therapies. As healthcare providers and patients seek innovative alternatives to traditional interventions, understanding the regulatory landscape surrounding cold atmospheric plasma technology becomes essential for informed medical decision-making.
The journey toward FDA clearance for cold plasma devices has been characterized by rigorous scientific validation, comprehensive safety testing, and demonstrated clinical efficacy. These regulatory approvals signal the technology’s potential to transform various medical specialties while maintaining the highest standards of patient safety and therapeutic effectiveness.
Understanding Cold Plasma Technology and FDA Regulation
What is Cold Atmospheric Plasma?
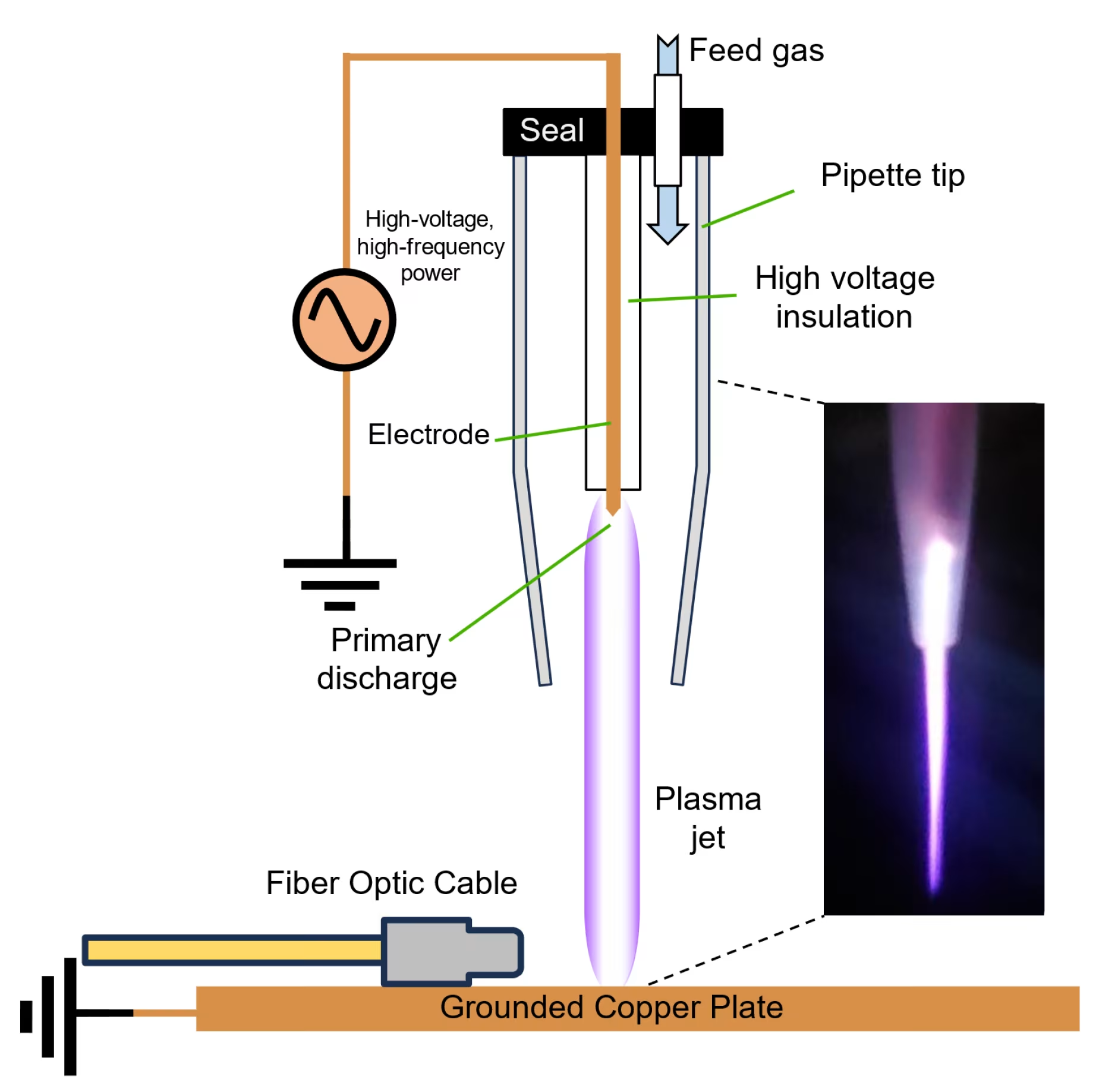
Cold atmospheric plasma (CAP) represents the fourth state of matter, created when energy is applied to gas molecules at room temperature, causing ionization without generating excessive heat[1]. Unlike traditional thermal plasma that operates at extreme temperatures, cold plasma maintains temperatures between 24°C to 30°C, making it safe for direct medical applications.
The technology generates a complex mixture of reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, electric fields, and UV photons. These components work synergistically to create therapeutic effects including antimicrobial action, tissue regeneration, and cellular modulation without causing thermal damage to healthy tissues.
FDA Regulatory Framework for Cold Plasma Devices
The FDA classifies cold plasma devices primarily under Class II medical devices, requiring premarket notification through the 510(k) pathway[2]. This classification reflects the moderate risk profile of these devices while acknowledging their therapeutic potential when properly regulated.
The regulatory process involves demonstrating substantial equivalence to legally marketed predicate devices, providing comprehensive safety and efficacy data, and meeting specific performance standards. Manufacturers must also comply with Quality System Regulations (QSR) and maintain detailed documentation throughout the product lifecycle.
Timeline of FDA Approvals for Cold Plasma Devices
Early Research and Development Phase (2008-2019)
The foundation for FDA approvals for cold plasma devices was established through extensive preclinical research beginning around 2008. During this period, researchers focused on understanding the fundamental mechanisms of cold plasma interaction with biological tissues and establishing safety parameters for human application[3].
Initial studies demonstrated cold plasma’s selective cytotoxicity against cancer cells while preserving healthy tissue integrity. This research established the scientific basis for subsequent clinical trials and regulatory submissions, paving the way for the first FDA-approved clinical applications.
First Clinical Trial Approval (2019)
A pivotal moment in FDA approvals for cold plasma devices occurred in August 2019 when the FDA approved the first clinical trial of cold plasma technology for cancer treatment[4]. This approval marked the beginning of systematic clinical evaluation of cold plasma devices in human subjects.
The approved trial focused on treating residual cancerous tissues following surgical tumor removal, addressing a critical unmet medical need in oncology. This milestone demonstrated the FDA’s recognition of cold plasma technology’s therapeutic potential and established a precedent for future approvals.
Recent FDA Clearances and Market Developments
Canady Helios Cold Plasma Ablation System
On May 7, 2024, US Medical Innovations LLC received FDA 510(k) clearance (K240297) for the Canady Helios Cold Plasma Ablation System[5]. This system is indicated for gas-enhanced ablation of soft tissue using helium-based plasma during surgical procedures.
The Canady Helios system represents a significant advancement in surgical oncology, utilizing a plasma jet that operates at non-thermal temperatures to target cancer cells while preserving surrounding healthy tissue. Clinical trials demonstrated exceptional safety and efficacy profiles with up to 80% non-local recurrence rates and overall survival rates reaching 86%[6].
MIRARI Cold Plasma System FDA Clearance
In November 2024, the MIRARI Cold Plasma System, developed by General Vibronics and commercialized by Mirari Doctor, received FDA clearance (K242553)[7]. This handheld device represents the first portable cold plasma system cleared for providing heating to elevate tissue temperature for temporary relief of pain, muscle spasms, and increased local circulation.
The MIRARI system utilizes proprietary nitric oxide-based cold plasma technology, distinguishing it from traditional ROS-based systems. This innovative approach offers enhanced safety profiles while maintaining therapeutic efficacy across various medical applications.
Technical Specifications of FDA-Approved Cold Plasma Devices
| Device Parameter | Canady Helios System | MIRARI Cold Plasma System |
|---|---|---|
| Operating Temperature | 24°C to 30°C | Room temperature (non-thermal) |
| Power Output | Variable (system-dependent) | 2-4W adjustable |
| Treatment Duration | 5-7 minutes per application | 15 minutes maximum per session |
| Frequency Range | Helium-based plasma generation | 80 kHz monopolar RF |
| Application Method | Intraoperative surgical margin treatment | Handheld direct application |
| FDA Clearance Number | K240297 | K242553 |
| Primary Indication | Soft tissue ablation during surgery | Pain relief, muscle spasm reduction |
Clinical Applications and Benefits of FDA-Approved Cold Plasma Devices
| Application Area | Treatment Benefits | Session Duration | Clinical Outcomes |
|---|---|---|---|
| Surgical Oncology | Tumor margin ablation, reduced recurrence | 5-7 minutes intraoperatively | 80% non-local recurrence rate |
| Pain Management | Non-invasive pain relief, muscle relaxation | 15 minutes per session | Significant pain reduction |
| Wound Healing | Accelerated tissue repair, antimicrobial action | 10-20 minutes per treatment | Enhanced healing rates |
| Circulation Enhancement | Improved blood flow, tissue oxygenation | 15-20 minutes per session | Measurable circulation improvement |
| Infection Control | Broad-spectrum antimicrobial effects | Variable based on application | Effective pathogen reduction |
| Tissue Regeneration | Cellular stimulation, collagen production | 10-30 minutes per treatment | Enhanced tissue quality |
Oncological Applications
FDA-approved cold plasma devices have shown particular promise in oncological applications. The Canady Helios system’s ability to target microscopic residual cancer cells while preserving healthy tissue represents a significant advancement in surgical oncology[8].
Clinical trials have demonstrated that cold plasma treatment of surgical margins can significantly reduce local recurrence rates while maintaining excellent safety profiles. The technology’s selective cytotoxicity against cancer cells, combined with its ability to stimulate immune responses, offers a multifaceted approach to cancer treatment.
Pain Management and Rehabilitation
The MIRARI Cold Plasma System’s FDA clearance for pain management applications opens new possibilities for non-pharmacological pain relief. The device’s ability to provide temporary relief of pain and muscle spasms through controlled tissue heating offers an alternative to traditional pain management approaches[9].
Healthcare providers can utilize this technology for various pain conditions, including chronic pain, muscle tension, and circulation-related discomfort. The non-invasive nature of the treatment makes it suitable for patients who may not be candidates for more aggressive interventions.
Safety Considerations and Regulatory Compliance
FDA Safety Requirements
FDA approvals for cold plasma devices include stringent safety requirements designed to protect patients while ensuring therapeutic efficacy. These requirements encompass device design, manufacturing quality, clinical testing, and post-market surveillance[10].
Key safety considerations include temperature control mechanisms, treatment duration limits, operator training requirements, and contraindication guidelines. The FDA requires comprehensive risk assessment and mitigation strategies for all approved cold plasma devices.
Clinical Safety Profiles
Clinical trials supporting FDA approvals for cold plasma devices have consistently demonstrated favorable safety profiles. Common side effects are typically mild and temporary, including slight skin redness or sensitivity at the treatment site[11].
The non-thermal nature of cold plasma technology significantly reduces the risk of burns or thermal injury compared to traditional thermal therapies. This safety advantage makes cold plasma devices suitable for a broader range of patients and applications.
Contraindications and Precautions
While FDA-approved cold plasma devices have excellent safety profiles, certain contraindications and precautions must be observed. These may include pregnancy, active infections at the treatment site, certain medical implants, and specific medical conditions.
Healthcare providers must conduct thorough patient assessments before treatment and follow manufacturer guidelines for safe device operation. Proper training and certification are essential for optimal patient outcomes and regulatory compliance.
Comparing FDA-Approved Cold Plasma Devices to Traditional Treatments
Advantages Over Conventional Therapies
FDA-approved cold plasma devices offer several advantages over traditional treatment modalities. The non-invasive nature of cold plasma therapy eliminates many risks associated with surgical procedures while providing comparable or superior therapeutic outcomes[12].
Unlike pharmaceutical interventions that may cause systemic side effects, cold plasma therapy provides localized treatment with minimal adverse effects. This targeted approach reduces the risk of drug interactions and systemic complications.
Cost-Effectiveness and Accessibility
The approval of portable cold plasma devices like the MIRARI system enhances treatment accessibility and cost-effectiveness. Healthcare providers can offer advanced plasma therapy in outpatient settings without requiring specialized facilities or extensive infrastructure.
This accessibility improvement enables broader patient access to innovative cold plasma treatments while reducing overall healthcare costs through shorter treatment times and reduced need for repeat interventions.
Future Prospects for FDA Approvals for Cold Plasma Devices
Emerging Applications
The success of current FDA approvals for cold plasma devices paves the way for expanded applications in various medical fields. Ongoing research explores cold plasma’s potential in dermatology, dentistry, ophthalmology, and regenerative medicine[13].
Future FDA submissions are likely to include devices targeting specific medical conditions such as diabetic ulcers, skin disorders, and antimicrobial applications. The growing body of clinical evidence supports the expansion of cold plasma technology into new therapeutic areas.
Technological Advancements
Continued innovation in cold plasma technology is driving the development of more sophisticated devices with enhanced capabilities. Future FDA approvals may include devices with improved targeting mechanisms, automated treatment protocols, and integrated monitoring systems.
The integration of artificial intelligence and machine learning into cold plasma devices may enable personalized treatment protocols and real-time optimization of therapeutic parameters, further enhancing patient outcomes and safety.
Regulatory Pathways for Cold Plasma Device Approval
510(k) Premarket Notification Process
Most FDA approvals for cold plasma devices follow the 510(k) premarket notification pathway, which requires demonstration of substantial equivalence to legally marketed predicate devices. This process typically takes 90 days and involves comprehensive documentation of device safety and efficacy[14].
Manufacturers must provide detailed technical information, clinical data, and risk analysis to support their 510(k) submissions. The FDA reviews this information to ensure that new devices meet established safety and effectiveness standards.
Premarket Approval (PMA) Requirements
For higher-risk cold plasma devices or novel applications, the FDA may require Premarket Approval (PMA), which involves more extensive clinical trials and regulatory review. This pathway ensures that innovative devices meet the highest standards of safety and efficacy.
The PMA process includes comprehensive clinical studies, manufacturing quality assessments, and ongoing post-market surveillance requirements. While more rigorous than 510(k), PMA approval provides greater regulatory certainty for breakthrough technologies.
Quality Management and Manufacturing Standards
ISO 13485 Compliance
FDA-approved cold plasma device manufacturers must comply with ISO 13485 quality management standards, ensuring consistent product quality and regulatory compliance. This international standard specifically addresses quality management systems for medical device manufacturing[15].
The MIRARI Cold Plasma System, developed by General Vibronics, exemplifies this commitment to quality through comprehensive quality management systems and rigorous manufacturing controls. The company’s adherence to international standards supports its FDA clearance and global market access.
Good Manufacturing Practices (GMP)
FDA approvals for cold plasma devices require compliance with Good Manufacturing Practices, ensuring that devices are consistently produced according to quality standards. GMP compliance encompasses all aspects of production, from raw material procurement to final product release.
Regular inspections and audits verify ongoing compliance with GMP requirements, maintaining the integrity of approved cold plasma devices throughout their commercial lifecycle.
Patient-Focused Frequently Asked Questions
Are FDA-approved cold plasma devices safe for all patients?
FDA-approved cold plasma devices have excellent safety profiles when used according to approved indications and manufacturer guidelines. However, certain patients may not be suitable candidates for cold plasma therapy, including pregnant women, patients with active infections at the treatment site, or those with specific medical implants. Healthcare providers conduct thorough assessments to determine patient suitability and ensure safe treatment delivery.
How do FDA-approved cold plasma devices compare to other pain management options?
FDA-approved cold plasma devices like the MIRARI system offer several advantages over traditional pain management approaches. Unlike pharmaceutical interventions that may cause systemic side effects, cold plasma provides localized treatment with minimal adverse effects. The non-invasive nature eliminates risks associated with injections or surgical procedures, while the absence of drug interactions makes it suitable for patients taking multiple medications.
What should patients expect during cold plasma treatment with FDA-approved devices?
Patients typically experience minimal discomfort during cold plasma treatment with FDA-approved devices. The therapy feels like gentle warmth or mild tingling at the treatment site. Sessions usually last 15 minutes or less, and patients can resume normal activities immediately after treatment. Some patients may notice slight redness at the treatment site, which typically resolves within a few hours.
How long do the effects of FDA-approved cold plasma treatment last?
The duration of therapeutic effects from FDA-approved cold plasma treatment varies depending on the specific condition being treated and individual patient factors. For pain management applications, patients often experience relief that lasts several hours to days following treatment. Multiple sessions may be recommended to achieve optimal and sustained results, with treatment frequency determined by healthcare providers based on patient response and clinical needs.
Can FDA-approved cold plasma devices be used at home?
Currently, FDA-approved cold plasma devices are designed for professional use by trained healthcare providers in clinical settings. The MIRARI Cold Plasma System and other approved devices require proper training and certification for safe operation. While the technology is portable and user-friendly, professional oversight ensures optimal treatment outcomes and patient safety. Patients interested in cold plasma therapy should consult with qualified healthcare providers who have access to FDA-approved devices.
Medical Disclaimer: This information is for educational purposes only and should not replace professional medical advice. Always consult with qualified healthcare providers before starting any new treatment. Individual responses to cold plasma therapy may vary, and treatment decisions should be based on comprehensive medical evaluation and professional judgment.
References
- Mirari Doctor. (2024). Cold Plasma and FDA Approvals. https://miraridoctor.com/fda-approvals-for-cold-plasma-devices/
- US Food and Drug Administration. (2024). 510(k) Premarket Notification Database – MIRARI Cold Plasma System. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K242553
- Dubey, S.K., et al. (2022). Emerging innovations in cold plasma therapy against cancer. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC9420777/
- News Medical. (2019). FDA approves cold atmospheric plasma technology for first-ever use in clinical trial. https://www.news-medical.net/news/20190820/FDA-approves-cold-atmospheric-plasma-technology-for-first-ever-use-in-clinical-trial.aspx
- US Food and Drug Administration. (2024). K240297 – Canady Helios Cold Plasma Ablation System. https://www.accessdata.fda.gov/cdrh_docs/pdf24/K240297.pdf
- BioSpace. (2024). US Medical Innovations Secures FDA Clearance for Canady Helios Cold Plasma Ablation System. https://www.biospace.com/us-medical-innovations-secures-fda-clearance-for-canady-helios-cold-plasma-ablation-system
- US Food and Drug Administration. (2024). K242553 – MIRARI Cold Plasma System. https://www.accessdata.fda.gov/cdrh_docs/pdf24/K242553.pdf
- Medical Device Network. (2024). FDA issues 510k clearance for US Medical’s ablation system. https://www.medicaldevice-network.com/news/fda-510k-us-medical-ablation/
- Mirari Doctor. (2024). Is Cold Plasma FDA Approved? https://miraridoctor.com/is-cold-plasma-fda-approved/
- MPO Magazine. (2024). FDA Clears Canady Helios Cold Plasma Ablation System. https://www.mpo-mag.com/breaking-news/fda-clears-canady-helios-cold-plasma-ablation-system/
- Business Wire. (2024). US Medical Innovations Secures FDA Clearance for Canady Helios Cold Plasma Ablation System. https://www.businesswire.com/news/home/20240507587574/en/US-Medical-Innovations-Secures-FDA-Clearance-for-Canady-Helios-Cold-Plasma-Ablation-System
- NS Medical Devices. (2024). US Medical gets FDA nod for Canady Helios cold plasma ablation system. https://www.nsmedicaldevices.com/news/us-medical-canady-helios-cold-plasma-ablation-system/
- MDPI. (2024). Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control. https://www.mdpi.com/2571-6182/7/1/14
- US Food and Drug Administration. (2025). 510(k) Premarket Notification. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm
- George Washington University. Cold Plasma Ablation System Developed with Professor Keidar’s Expertise Gains FDA Clearance. https://engineering.gwu.edu/cold-plasma-ablation-system-developed-professor-keidars-expertise-gains-fda-clearance
Related articles
Made in USA