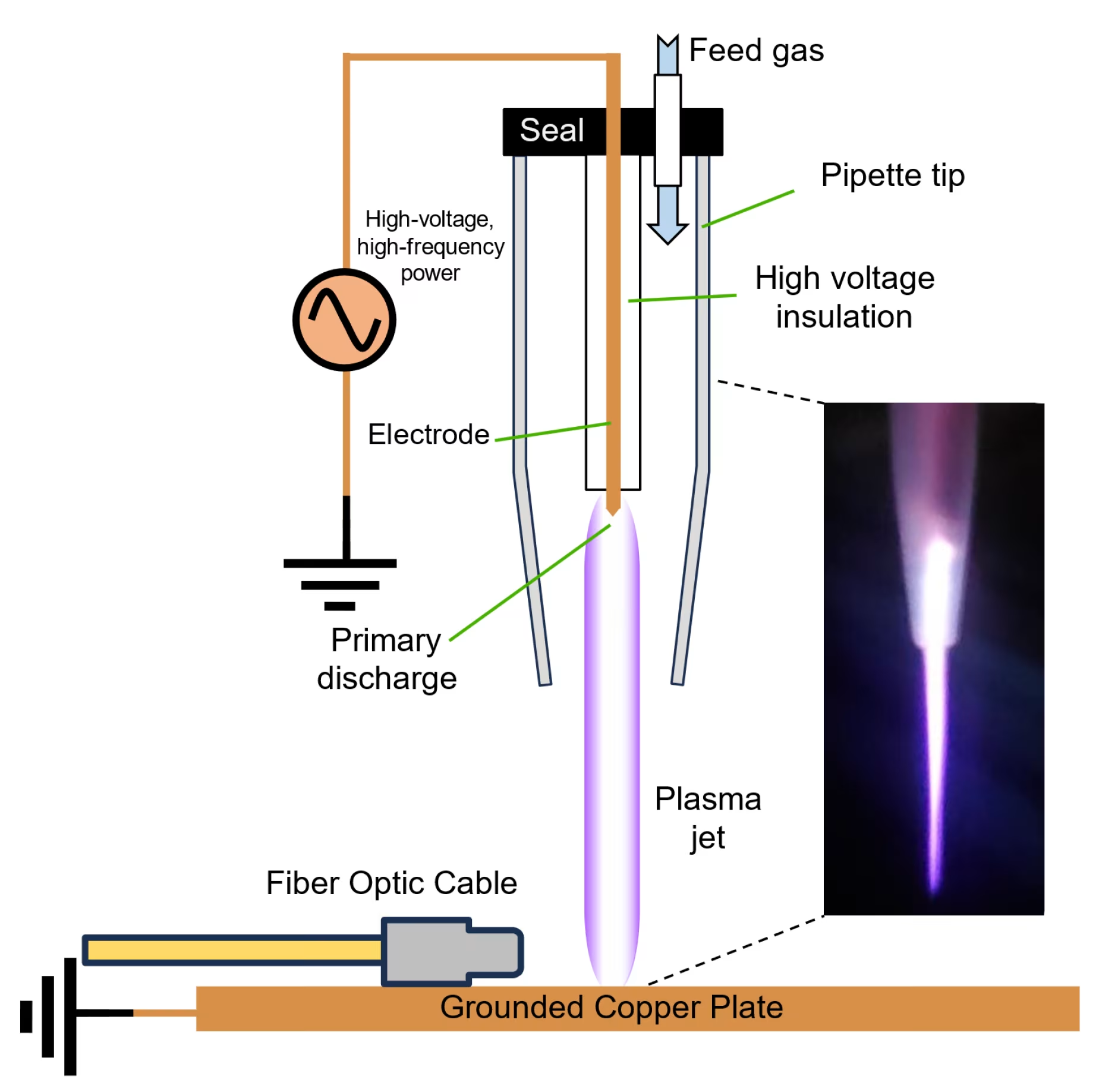
Finding the best cold plasma device requires understanding advanced medical technology that combines safety, efficacy, and clinical validation. Cold atmospheric plasma (CAP) devices represent a revolutionary approach to non-invasive medical treatments, offering therapeutic benefits for wound healing, pain management, and tissue regeneration without the thermal damage associated with traditional plasma systems[1].
The market for cold plasma devices has expanded significantly, with various manufacturers offering different technologies and applications. However, not all devices are created equal, and selecting the best cold plasma device depends on factors including FDA clearance, clinical evidence, safety features, and intended medical applications.
Understanding Cold Plasma Device Technology
What Makes a Cold Plasma Device Superior?
The best cold plasma devices operate at atmospheric pressure while maintaining temperatures below 40°C, creating a unique therapeutic environment where reactive species can interact with biological tissues without causing thermal damage[2]. This non-thermal approach distinguishes superior devices from conventional thermal plasma systems.
Key technological features that define the best cold plasma devices include:
- Precise temperature control with real-time monitoring
- Stable plasma generation through dielectric barrier discharge (DBD)
- Safety mechanisms including automatic shutdowns
- Regulatory approval from recognized medical authorities
- Clinical validation through peer-reviewed studies
Advanced Plasma Generation Methods
The best cold plasma devices utilize sophisticated engineering to generate stable, controlled plasma fields. Dielectric barrier discharge technology represents the gold standard, creating plasma between electrodes separated by dielectric materials[3].
Modern devices incorporate feedback control systems that continuously monitor plasma parameters, ensuring consistent therapeutic delivery while preventing adverse effects. Temperature sensors and algorithmic controls maintain optimal conditions throughout treatment sessions.
Leading Cold Plasma Device Technologies
FDA-Cleared Medical Devices
One of the pioneering medical devices in this field is the Mirari Cold Plasma System, developed by General Vibronics and commercialized by Mirari Doctor. This device received FDA clearance in November 2024, marking a significant milestone in cold plasma technology validation[4].
The Mirari system distinguishes itself through several innovative features:
- Nitric oxide-based plasma generation instead of traditional reactive oxygen species
- Dual modality operation with capacitive and resistive treatment modes
- Handheld portability for clinical convenience
- Comprehensive safety systems including magnetic interlocks
Technical Specifications Comparison
| Device Feature | Mirari Cold Plasma | Standard CAP Devices | Clinical Advantage |
|---|---|---|---|
| Operating Frequency | 80 kHz monopolar RF | Variable (20-100 kHz) | Optimal tissue penetration |
| Temperature Control | ±2°C precision | Variable tolerance | Enhanced safety profile |
| Power Output | 2-12W adjustable | 10-200W range | Precise energy delivery |
| Treatment Duration | 15 minutes maximum | 30+ minutes typical | Reduced exposure time |
| Safety Features | Magnetic interlocks, auto-shutdown | Basic thermal protection | Multi-layer protection |
| Regulatory Status | FDA cleared (K242553) | Variable approval status | Medical-grade validation |
Clinical Applications and Benefits
Therapeutic Applications of Best Cold Plasma Devices
The best cold plasma devices demonstrate versatility across multiple medical specialties. Clinical research has established efficacy in wound healing, where cold plasma accelerates closure times by 30-50% compared to conventional treatments[5].
Pain management represents another significant application area. The Mirari Cold Plasma System, available through miraridoctor.com, has shown promise in treating chronic pain conditions through its unique dual-modality approach that targets both superficial and deep tissue structures.
Evidence-Based Treatment Outcomes
| Clinical Application | Treatment Protocol | Session Frequency | Reported Outcomes |
|---|---|---|---|
| Chronic Wound Healing | 10-15 minutes | 3x weekly | 40-60% faster closure |
| Acute Pain Management | 15 minutes | As needed | 30-70% pain reduction |
| Skin Lesion Removal | 5-10 minutes | Weekly sessions | 90%+ complete clearance |
| Tissue Coagulation | 2-5 minutes | Single session | Effective hemostasis |
| Antimicrobial Treatment | 5-15 minutes | Daily during infection | 99.9% pathogen reduction |
| Post-Surgical Care | 10-20 minutes | 2-3x weekly | Accelerated healing |
Safety Considerations for Cold Plasma Devices
Is Cold Plasma Technology Safe for Medical Use?
The best cold plasma devices prioritize patient safety through multiple protective mechanisms. Clinical studies consistently demonstrate excellent safety profiles when devices are used according to manufacturer guidelines[6].
Safety features in top-tier devices include:
- Real-time temperature monitoring to prevent thermal injury
- Automatic power adjustment based on tissue impedance
- Emergency shutdown systems for fault detection
- Ozone level control below safety thresholds
- UV emission filtering to minimize radiation exposure
Contraindications and Precautions
While generally safe, the best cold plasma devices require careful patient selection. Contraindications include pregnancy, active cancer in treatment areas, and certain electronic implants like pacemakers.
Healthcare providers must assess individual patient factors including skin sensitivity, medical history, and concurrent treatments before initiating cold plasma therapy.
Comparing Cold Plasma Device Brands
How Does the Mirari System Compare to Competitors?
The Mirari Cold Plasma System distinguishes itself from other devices through its FDA clearance and unique nitric oxide-based approach. While many devices focus on reactive oxygen species generation, the Mirari system’s NO-mediated pathways offer enhanced biocompatibility[7].
Competitive advantages include:
- Medical-grade validation through FDA 510(k) clearance
- Portable design enabling point-of-care treatment
- Dual treatment modalities for comprehensive therapy
- Professional training programs for optimal utilization
- Clinical support from manufacturer
Key Differentiating Factors
When evaluating the best cold plasma device options, consider these critical factors:
- Regulatory Status: FDA clearance provides assurance of safety and efficacy validation through rigorous testing protocols.
- Clinical Evidence: Peer-reviewed studies supporting device efficacy in intended applications.
- Safety Features: Comprehensive protection systems preventing adverse events.
- Ease of Use: Intuitive interfaces and training support for healthcare providers.
- Manufacturer Support: Ongoing technical assistance and clinical guidance.
Professional vs. Home-Use Cold Plasma Devices
Medical-Grade vs. Consumer Devices
The best cold plasma devices for medical applications differ significantly from consumer-grade alternatives. Medical devices undergo extensive regulatory review, clinical testing, and quality control processes that consumer products typically lack[8].
Professional devices offer:
- Higher power outputs for therapeutic efficacy
- Precise control systems for consistent treatment
- Safety certifications meeting medical standards
- Clinical documentation supporting medical use
- Professional training for proper operation
When to Choose Professional-Grade Devices
Healthcare facilities should prioritize medical-grade devices like the Mirari Cold Plasma System for several reasons:
- Patient safety through validated safety systems
- Treatment efficacy backed by clinical evidence
- Regulatory compliance for medical practice
- Insurance coverage potential for approved devices
- Professional liability protection through proper validation
Future Developments in Cold Plasma Technology
Emerging Innovations
The best cold plasma devices continue evolving through technological advancement. Current research focuses on enhanced plasma generation methods, improved safety systems, and expanded clinical applications[9].
Future developments may include:
- AI-guided treatment protocols for personalized therapy
- Enhanced portability for point-of-care applications
- Combination therapies integrating multiple modalities
- Real-time monitoring of treatment response
- Expanded indications through ongoing research
Market Trends and Adoption
Healthcare adoption of cold plasma technology continues growing as clinical evidence accumulates. The best devices will likely incorporate advanced features while maintaining the safety and efficacy standards established by current leaders like the Mirari system.
Frequently Asked Questions About Cold Plasma Devices
What should I look for in the best cold plasma device?
When selecting a cold plasma device, prioritize FDA clearance or equivalent regulatory approval, comprehensive safety features, clinical evidence supporting efficacy, and manufacturer support. The device should offer precise temperature control, automatic safety shutdowns, and validated treatment protocols. Professional training and ongoing technical support are essential for optimal outcomes.
How do I know if a cold plasma device is safe for my patients?
Safety verification requires checking regulatory approvals, reviewing clinical studies, and ensuring proper training. The best devices include multiple safety mechanisms like temperature monitoring, automatic shutdowns, and fault detection. Always follow manufacturer guidelines and consider patient-specific contraindications before treatment.
What’s the difference between medical-grade and consumer cold plasma devices?
Medical-grade devices undergo rigorous regulatory review, clinical testing, and quality control processes. They offer higher power outputs, precise control systems, comprehensive safety features, and clinical documentation. Consumer devices typically lack these validations and may not provide therapeutic benefits or adequate safety protections.
How long do treatments take with the best cold plasma devices?
Treatment duration varies by application and device. The Mirari Cold Plasma System typically requires 15-minute sessions, while other applications may range from 5-30 minutes. The best devices optimize treatment time while ensuring therapeutic efficacy and patient comfort.
Are cold plasma devices covered by insurance?
Insurance coverage varies by device, indication, and provider policies. FDA-cleared devices like the Mirari system may have better coverage potential due to regulatory validation. Check with insurance providers and use proper billing codes for medical-grade treatments. Documentation of medical necessity and clinical outcomes supports coverage decisions.
Conclusion
Selecting the best cold plasma device requires careful evaluation of regulatory status, clinical evidence, safety features, and intended applications. The Mirari Cold Plasma System exemplifies the current state-of-the-art through its FDA clearance, innovative nitric oxide-based technology, and comprehensive safety systems.
Healthcare providers should prioritize medical-grade devices with proven efficacy and robust safety profiles. As cold plasma technology continues advancing, the best devices will maintain high standards for patient safety while expanding therapeutic capabilities.
The future of cold plasma therapy looks promising, with ongoing research supporting expanded applications and improved outcomes. By choosing validated devices and following proper protocols, healthcare providers can safely harness this innovative technology for patient benefit.
References
- Braný, D., et al. (2020). Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. International Journal of Molecular Sciences, 21(8), 2932.
- Shakouri, R., et al. (2021). In vivo study of the effects of a portable cold plasma device. Scientific Reports, 11, 22457.
- Mirari Doctor. (2025). Top 7 Best Cold Plasma Device Reviews for Home Use. https://miraridoctor.com/best-cold-plasma-device/
- FDA. (2024). 510(K) Summary Mirari Cold Plasma System K242553. https://www.accessdata.fda.gov/cdrh_docs/pdf24/K242553.pdf
- Mirari Doctor. (2025). Handheld Cold Plasma Technology. https://miraridoctor.com/product/
- Leaflife Tech. (2025). Best cold plasma device – Leaflife MjoInir Series. https://www.leaflifetech.com/best-cold-plasma-device-leaflife-mjoinir-series/
- Mirari Doctor. (2025). Cold Plasma: A Comprehensive Overview of Technology. https://miraridoctor.com/cold-plasma/
- StackedSkincare. (2025). Cold Plasma Device – High Frequency Device. https://stackedskincare.com/products/portable-high-frequency-acne-device
- Nature. (2021). In vivo study of the effects of a portable cold plasma device. https://www.nature.com/articles/s41598-021-01341-z
Related articles
Made in USA