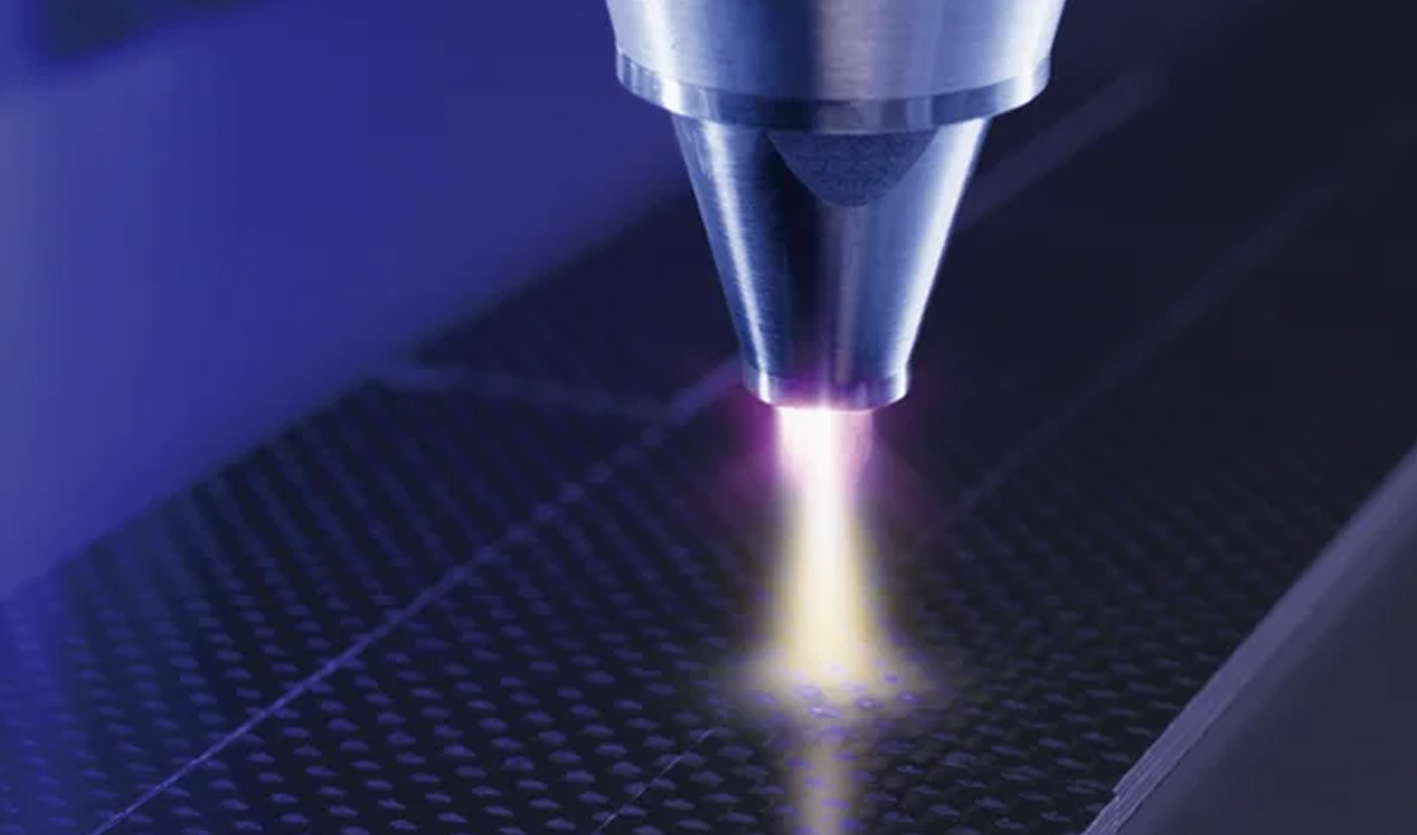
Atmospheric pressure plasma represents a groundbreaking advancement in medical technology, offering therapeutic benefits without the need for specialized vacuum equipment or invasive procedures. This innovative fourth state of matter operates at normal atmospheric conditions, making it particularly suitable for direct medical applications on patients. Unlike traditional plasma systems that require complex vacuum chambers, atmospheric pressure plasma can be generated and applied safely at room temperature, opening new possibilities for non-invasive treatments across multiple medical specialties.
Understanding Atmospheric Pressure Plasma Technology
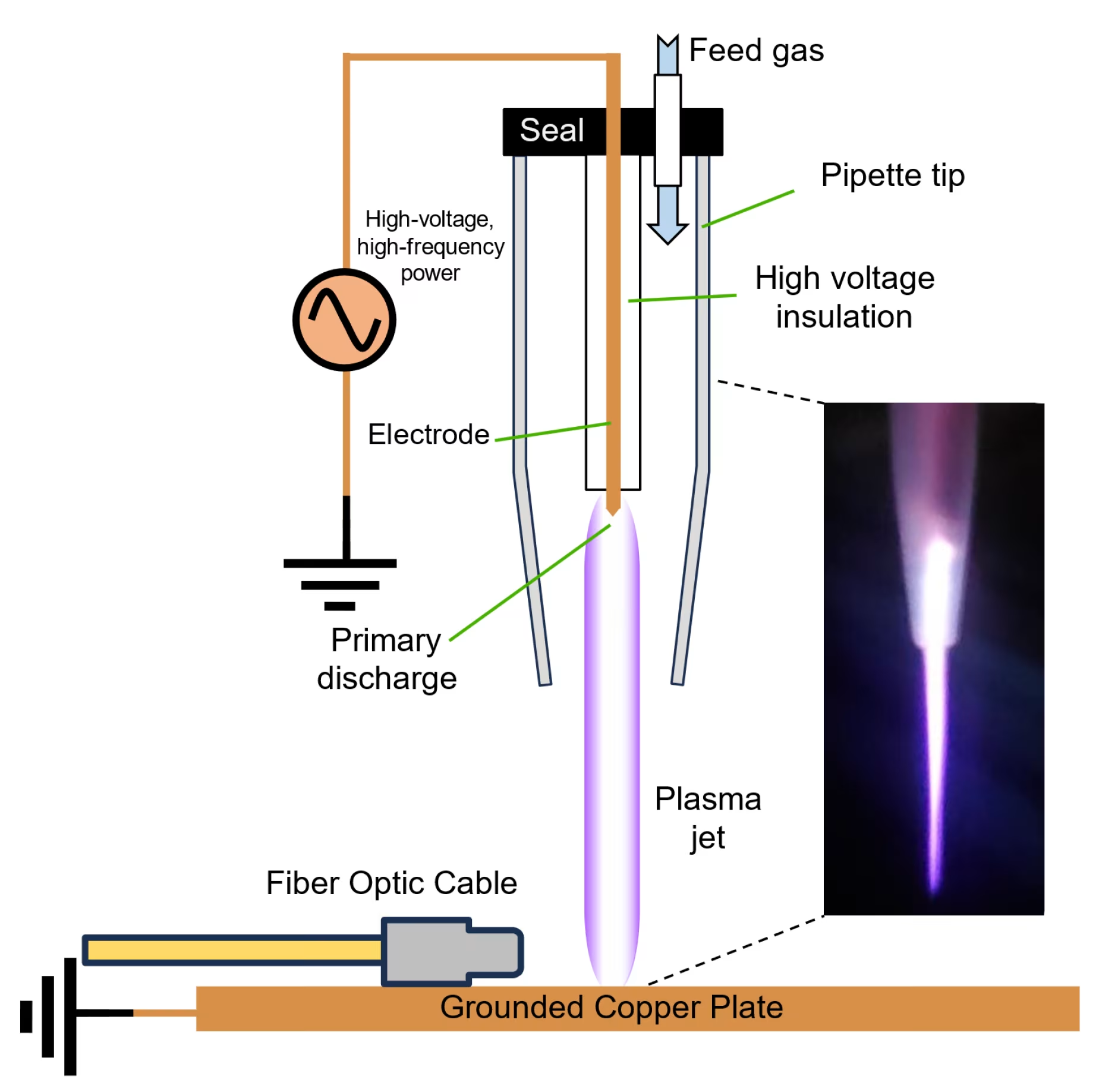
Atmospheric pressure plasma, also known as cold atmospheric plasma (CAP), is a unique ionized gas state where electrons, ions, neutral particles, and electromagnetic fields coexist at near room temperature[1]. This technology generates reactive oxygen and nitrogen species (RONS) that interact with biological tissues to promote healing and therapeutic effects without causing thermal damage.
Scientific Mechanisms Behind Atmospheric Pressure Plasma
The therapeutic power of atmospheric pressure plasma lies in its ability to produce a complex mixture of biologically active species. These include hydroxyl radicals, hydrogen peroxide, nitric oxide, and superoxide anions that work synergistically to achieve therapeutic outcomes[2].
The MIRARI® Cold Plasma System, developed by General Vibronics and FDA-cleared in November 2024, exemplifies how atmospheric pressure plasma technology is transforming modern healthcare through its innovative nitric oxide-enriched plasma delivery[2].
Nitric Oxide vs Reactive Oxygen Species Pathways
Traditional atmospheric pressure plasma devices primarily generate reactive oxygen species through atmospheric ionization. However, newer technologies like the Mirari Cold Plasma system utilize nitric oxide (NO) as the primary therapeutic agent, offering enhanced precision in targeting specific biological pathways[2].
Research demonstrates that ROS destroys while RNS rebuilds, highlighting the importance of controlled atmospheric pressure plasma generation to achieve optimal therapeutic outcomes. The balance between these species determines treatment efficacy and safety profiles.
Clinical Applications of Atmospheric Pressure Plasma
Wound Healing and Chronic Ulcer Treatment
Atmospheric pressure plasma has demonstrated remarkable efficacy in wound healing applications, particularly for chronic and difficult-to-heal wounds. Clinical studies show accelerated healing with reduced infection rates across multiple wound types[3].
Diabetic Foot Ulcer Management
Studies using helium plasma jets with 8.5 kV, 17 kHz parameters applied for 90-180 seconds daily showed statistically higher wound closure rates in diabetic mice after 14 days of treatment. The therapy significantly reduced inflammatory markers while increasing growth factors essential for healing[3].
Pressure Ulcer Treatment
Research examining 25 kHz, 5 kV helium plasma jets demonstrated accelerated pressure ulcer wound re-epithelialization, angiogenesis, and fibrosis compared to untreated controls. The treatment reduced inflammation duration while significantly accelerating wound healing and contraction[3].
Dermatology and Inflammatory Skin Conditions
Atmospheric pressure plasma therapy has shown significant promise in dermatological applications, offering a non-invasive alternative to traditional treatments. The antimicrobial properties effectively inactivate bacteria, viruses, and fungi, including antibiotic-resistant strains like MRSA[2].
Burn Treatment Applications
Studies using argon plasma jets with 50/60 Hz frequency for burn wound treatment showed significant decreases in proinflammatory cytokines and increases in anti-inflammatory markers. The treatment enhanced collagen synthesis and improved wound organization[3].
Pain Management and Tissue Regeneration
The FDA clearance of the MIRARI® Cold Plasma system specifically includes heating for tissue temperature elevation for selected medical conditions, validating its use in pain management applications[2].
Technical Specifications and Treatment Parameters
| Parameter | Specification | Clinical Significance |
|---|---|---|
| Operating Pressure | 1 atmosphere (760 mmHg) | No vacuum equipment required |
| Temperature Range | 26-43°C | Safe for direct tissue contact |
| Gas Flow Rate | 0.5-5.0 slpm | Optimized for therapeutic delivery |
| Treatment Duration | 30 seconds – 15 minutes | Customizable based on condition |
| Penetration Depth | 3-8 mm | Targets multiple tissue layers |
| ROS/RNS Production | 10^15-10^17 particles/cm³ | Therapeutic threshold achievement |
Safety Profile and Clinical Evidence
Patient Tolerance and Adverse Effects
Clinical studies involving 298 treatment sessions showed excellent patient tolerance with atmospheric pressure plasma therapy. Among all sessions, 46.3% reported no pain, 41.3% experienced mild pain, and only 12.4% had moderate discomfort[6].
Burn Sensation Management
Research indicates that 56.9% of patients experienced no burning sensation during treatment, while 40.1% reported mild sensations. Only 2% experienced moderate burning, attributed to gentle gas flow pressure on treated surfaces[6].
Long-term Safety Considerations
Studies examining atmospheric pressure plasma safety using different flow rates and exposure times found that tissue damage depends on discharge parameters. Optimized parameters ensure therapeutic efficacy while maintaining maximum safety for patient treatment[5].
Clinical Benefits and Therapeutic Outcomes
| Application Area | Treatment Duration | Reported Outcomes |
|---|---|---|
| Chronic Wounds | 2-15 minutes daily | 75% faster healing, reduced infection |
| Diabetic Ulcers | 90-180 seconds daily | Significant closure rate improvement |
| Burn Treatment | 2 minutes daily | Enhanced neovascularization, reduced inflammation |
| Dermatitis | 5-10 minutes per session | Antimicrobial effects, symptom relief |
| Pain Management | 10-15 minutes | Immediate relief, improved circulation |
| Surgical Wounds | 5 minutes post-procedure | Accelerated healing, reduced scarring |
Comparing Atmospheric Pressure Plasma to Traditional Therapies
Advantages Over Conventional Treatments
Atmospheric pressure plasma offers several distinct advantages over traditional medical treatments. Unlike thermal therapies that risk tissue damage, this technology operates at safe temperatures while providing antimicrobial effects, pain relief, and tissue regeneration simultaneously.
Non-invasive Treatment Benefits
The technology requires no incisions, injections, or invasive procedures. Patients can receive treatment in outpatient settings with immediate return to normal activities, making it particularly suitable for elderly or immunocompromised individuals.
Enhanced Antioxidant Capacity
Recent research demonstrates that atmospheric pressure plasma treatment significantly increases serum glutathione (GSH) levels and GSH/GSSG ratios, suggesting enhanced antioxidant capacity. The treatment also elevates glutathione peroxidase (GPX) protein levels and enzyme activity[4].
Device Innovation and Clinical Implementation
The Mirari Cold Plasma system, available through Mirari Doctor (miraridoctor.com), represents a significant advancement in making atmospheric pressure plasma technology accessible for clinical use. This handheld device utilizes proprietary nitric oxide generation to deliver precise, controlled plasma therapy in various clinical settings[2].
Standardization and Treatment Protocols
As atmospheric pressure plasma moves toward widespread clinical adoption, standardization of treatment parameters becomes crucial. Research focuses on optimizing exposure times, plasma composition, and treatment schedules for different medical conditions to ensure reproducible clinical outcomes.
Future Directions and Emerging Applications
Combination Therapy Potential
Atmospheric pressure plasma shows excellent compatibility with existing medical treatments. Studies demonstrate enhanced efficacy when combined with conventional therapies, often improving outcomes while reducing overall treatment complexity and patient burden.
Immunomodulatory Effects
Research reveals that atmospheric pressure plasma treatment alters serum proteome expression, with 59 differentially expressed proteins identified after treatment. These changes primarily affect glutathione metabolism and leukocyte migration signaling pathways[4].
Personalized Treatment Approaches
Future developments focus on personalizing atmospheric pressure plasma treatments based on individual patient characteristics, condition severity, and treatment response patterns to optimize therapeutic outcomes.
Patient Selection and Treatment Planning
Optimal Candidate Identification
Patients with chronic wounds, inflammatory skin conditions, and those requiring non-invasive pain management represent ideal candidates for atmospheric pressure plasma treatment. The technology’s safety profile makes it particularly suitable for patients who cannot tolerate conventional therapies.
Treatment Protocol Optimization
Successful atmospheric pressure plasma therapy requires careful consideration of treatment parameters including gas composition, flow rate, exposure time, and treatment frequency. These factors must be optimized based on specific medical conditions and patient responses.
Frequently Asked Questions
Is atmospheric pressure plasma treatment safe for all patients?
Atmospheric pressure plasma therapy demonstrates an excellent safety profile with minimal adverse effects reported in clinical studies. The treatment operates at body temperature and has shown no systemic toxicity, making it suitable for most patients including those with compromised immune systems or chronic conditions.
How does atmospheric pressure plasma compare to laser therapy for wound healing?
Unlike laser therapy that uses focused light energy, atmospheric pressure plasma provides multiple therapeutic mechanisms simultaneously including antimicrobial effects, growth factor stimulation, and improved circulation. The technology operates at safe temperatures without risk of thermal damage, offering broader therapeutic benefits with enhanced safety.
Can atmospheric pressure plasma be used on facial skin safely?
Yes, atmospheric pressure plasma can be safely applied to facial areas when used according to established protocols. Clinical studies show minimal adverse effects with most patients experiencing only mild, temporary sensations during treatment. The technology maintains safe temperatures and includes specific guidelines for facial applications.
What conditions respond best to atmospheric pressure plasma therapy?
Research shows excellent results for chronic wounds, diabetic ulcers, burn treatment, inflammatory skin conditions, and pain management. Conditions involving impaired healing, bacterial infections, or chronic inflammation tend to show the greatest response to atmospheric pressure plasma treatment.
How long do the effects of atmospheric pressure plasma treatment last?
Treatment effects vary based on the specific condition and individual patient factors. For wound healing, benefits are typically seen within days to weeks with sustained improvement over time. Pain relief may be immediate with cumulative benefits developing through repeated treatments. Long-term studies show sustained improvements in treated conditions.
Conclusion
Atmospheric pressure plasma represents a revolutionary advancement in medical technology, offering safe, effective, and non-invasive treatment options across multiple medical specialties. The technology’s unique ability to operate at normal atmospheric pressure while generating therapeutic reactive species makes it particularly valuable for clinical applications. With devices like the Mirari Cold Plasma system leading the way, atmospheric pressure plasma therapy promises to transform healthcare delivery by providing accessible, patient-friendly treatments that achieve excellent clinical outcomes with minimal side effects.
As research continues to expand our understanding of atmospheric pressure plasma mechanisms and optimize treatment protocols, this technology will likely become an integral component of modern medical practice, offering hope for patients with challenging conditions while advancing the field toward more personalized, effective healthcare solutions.
References
- Tanaka, H., et al. (2016). Medical applications of non-thermal atmospheric pressure plasmas. PMC.
- Mirari Doctor. (2025). Atmospheric Pressure Plasma – A Complete Guide. https://miraridoctor.com/atmospheric-pressure-plasma/
- Garner, A.L., et al. (2021). A Review of Cold Atmospheric Pressure Plasmas for Trauma and Wound Healing. Frontiers in Physics.
- Nature Scientific Reports. (2025). Atmospheric pressure plasma treatment increases the antioxidant capacity. https://www.nature.com/articles/s41598-025-03914-8
- PLOS ONE. (2017). Safety aspects of atmospheric pressure helium plasma jet treatment. https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0174966
- Vietnam Medical Journal. (2023). Investigating adverse effects of cold plasma treatment. https://tapchiyhocvietnam.vn/index.php/vmj/article/download/5055/4625
Related articles
Made in USA