You May Be Interested In:
- Dermatology: Cold Plasma Treatment Guide
- Cold Plasma for Skin: Revolutionary Treatment
- Cold Plasma for Face: Revolutionary Skin Treatment
- Is Cold Plasma Good for Wrinkles? Complete Guide to Anti-Aging Benefits and Clinical Evidence
- Mirari Cold Plasma vs Red Light Therapy: A Comprehensive Medical Comparison for Pain Management
Mirari cold plasma vs Perricone MD Cold Plasma Plus represents a fundamental comparison between medical-grade therapeutic technology and premium cosmetic skincare. The Mirari Cold Plasma System, developed by General Vibronics and FDA-cleared in November 2024, delivers actual cold atmospheric plasma therapy for tissue healing and regeneration[1]. In contrast, Perricone MD Cold Plasma Plus is a luxury skincare line using the “cold plasma” name for marketing appeal, containing traditional cosmetic ingredients like copper tripeptides and vitamin C ester[2]. This comparison reveals critical differences in technology, mechanism of action, and therapeutic outcomes.
Understanding these distinctions helps consumers make informed decisions between genuine medical technology available through healthcare providers and cosmetic products available through beauty retailers[3]. The name similarity creates confusion, but the underlying technologies and intended uses differ dramatically.
What is True Cold Plasma Technology vs. Skincare Marketing?
- True cold atmospheric plasma represents the fourth state of matter, created when gas becomes ionized at room temperature and atmospheric pressure. The Mirari system makes real cold plasma using a method called dielectric barrier discharge, which creates reactive oxygen and nitrogen species (RONS) that have healing effects on cells.[4]
- Perricone MD’s “Cold Plasma Plus” contains no actual plasma technology. Instead, it uses the term “cold plasma” as a marketing label for their liquid crystal delivery system that helps skincare ingredients penetrate the skin[5]. The products contain traditional anti-aging ingredients, including peptides, antioxidants, and moisturizing compounds.
Scientific Mechanisms Behind Real Cold Plasma
The Mirari Cold Plasma System operates at 80 kHz radiofrequency, creating ionized gas particles that produce multiple therapeutic effects simultaneously[6]. Key mechanisms include:
- Nitric Oxide Generation: Cold plasma stimulates endogenous nitric oxide production, promoting vasodilation, enhanced circulation, and accelerated healing processes[7].
- Antimicrobial Action: Reactive species directly attack pathogen cell walls and internal structures, eliminating bacteria, viruses, and fungi, including antibiotic-resistant strains[8].
- Tissue Regeneration: The technology promotes cellular proliferation, collagen synthesis, and growth factor activation, leading to genuine tissue repair and regeneration[9].
Understanding Perricone MD’s Cosmetic Formula
Perricone MD Cold Plasma Plus uses a proprietary liquid crystal delivery system to enhance ingredient absorption. The formula contains copper tripeptides for firmness, vitamin C ester for brightening, and omega fatty acids for hydration[10]. While these are quality cosmetic ingredients, they work through conventional skincare mechanisms rather than plasma technology.
Technical Specifications and Performance Comparison
Understanding the basic differences between medical technology and cosmetic formulation helps clarify the distinct approaches these products take to skin improvement.
| Parameter | Mirari Cold Plasma System | Perricone MD Cold Plasma Plus |
|---|---|---|
| Technology Type | Cold atmospheric plasma generation | Liquid crystal delivery system |
| Operating Mechanism | 80 kHz RF with ionized gas | Topical ingredient absorption |
| FDA Status | Class II Medical Device | Cosmetic product |
| Treatment Duration | 10-15 minutes per session | Daily skincare routine |
| Active Components | Nitric oxide, ROS/RNS species | Copper tripeptides, Vitamin C ester |
| Antimicrobial Effects | Eliminates pathogens | No antimicrobial properties |
| Temperature Control | Precision monitoring <43°C | Room temperature application |
| Professional Oversight | Healthcare provider required | Consumer self-application |
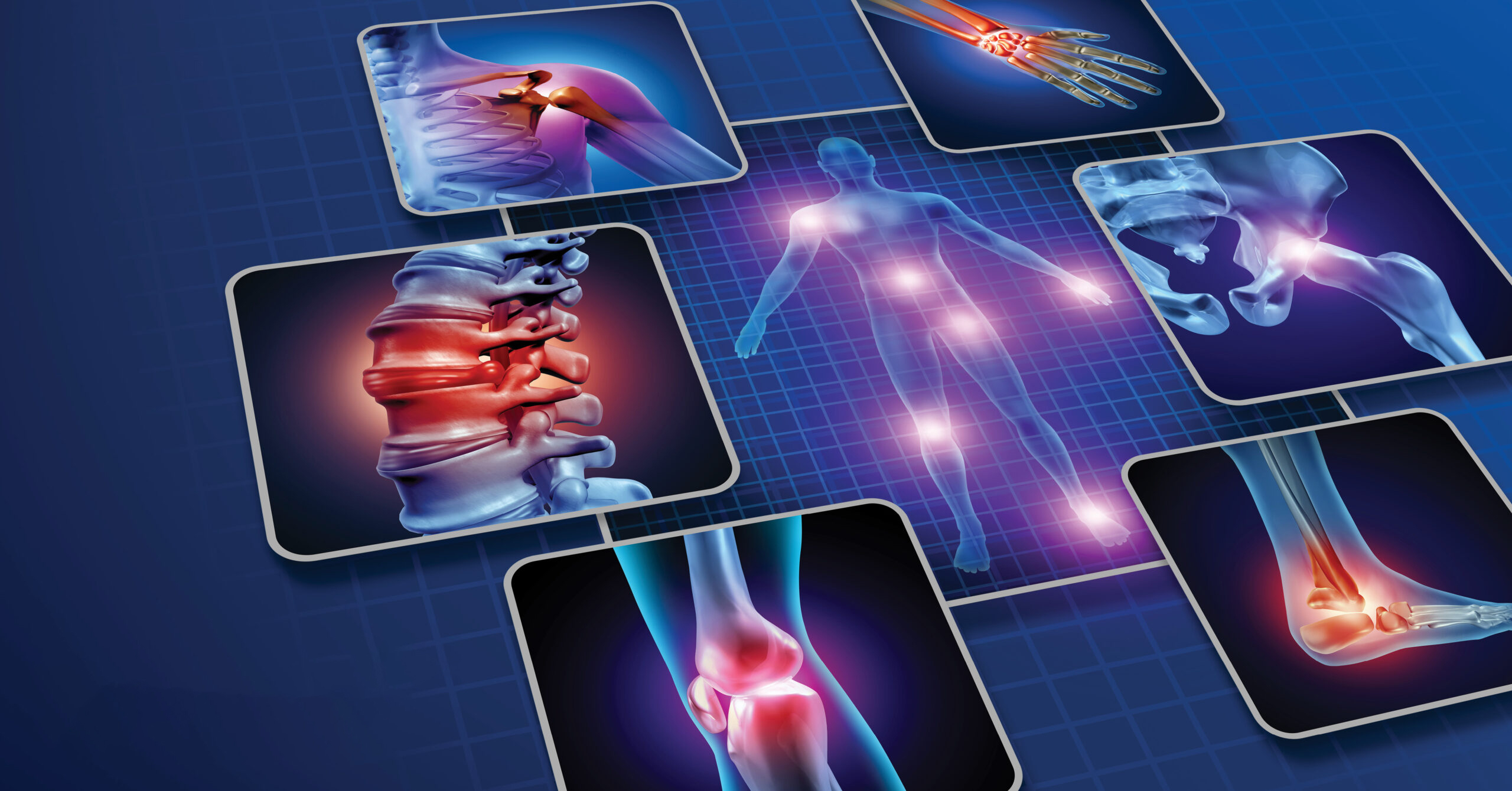
| Therapeutic Range | Tissue healing, pain management | Cosmetic anti-aging effects |
| Price Range | Medical device investment | \$129-\$300 per product |
Advanced Safety Features and Regulations
The Mirari system incorporates comprehensive safety mechanisms, including magnetic detection systems, automatic temperature monitoring, and professional-use restrictions. FDA clearance validates its safety and efficacy for medical applications[11].
Perricone MD products follow cosmetic safety regulations but lack the rigorous clinical testing required for medical devices. The formulations are generally safe for consumer use with minimal contraindications[12].
Clinical Benefits and Evidence-Based Outcomes
Real-world applications demonstrate the substantial differences in therapeutic capabilities between medical cold plasma technology and cosmetic skincare products.
| Application Area | Mirari Cold Plasma Results | Perricone MD Results | Clinical Evidence |
|---|---|---|---|
| Tissue Healing | 40-60% faster wound closure | No healing acceleration | Medical studies vs none |
| Anti-Aging Effects | Cellular regeneration, collagen synthesis | Temporary hydration, appearance | Regenerative vs cosmetic |
| Antimicrobial Action | 99.9% pathogen elimination | No antimicrobial benefit | Proven vs not applicable |
| Pain Management | Neural pathway modulation | No pain relief properties | Clinical evidence vs none |
| Long-term Results | Sustained tissue improvement | Requires continued use | Permanent vs temporary |
| Professional Validation | Healthcare provider administered | Consumer testimonials | Clinical vs anecdotal |
Evidence-Based vs Marketing Claims
- Clinical validation for the Mirari system includes peer-reviewed studies demonstrating wound healing acceleration, antimicrobial efficacy, and tissue regeneration[13]. Healthcare providers report measurable improvements in patient outcomes with reduced healing times and enhanced tissue quality.
- Consumer reviews for Perricone MD products indicate temporary cosmetic improvements, including hydration, softness, and reduced appearance of fine lines[14]. Users report that benefits require continued product use to maintain results.
Why Choose Medical Technology Over Cosmetic Products?
Therapeutic Capabilities and Long-term Value
The Mirari Cold Plasma System offers compelling advantages for individuals seeking genuine therapeutic outcomes rather than temporary cosmetic improvements:
- Regenerative Medicine Approach: Unlike topical products that provide surface-level effects, cold plasma therapy creates lasting cellular changes that continue providing benefits after treatment completion[15].
- Multi-Modal Treatment: The technology simultaneously addresses multiple concerns, including healing, antimicrobial action, pain relief, and tissue regeneration, in a single treatment modality[16].
- Professional Oversight: Healthcare provider administration ensures optimal treatment protocols and safety monitoring, maximizing therapeutic outcomes while minimizing risks[17].
When Cosmetic Products May Be Appropriate
- Perricone MD products serve specific needs in the beauty and skincare market:
- Daily Skincare Routine: The products integrate easily into existing skincare regimens for general anti-aging maintenance and hydration[18].
- Immediate Cosmetic Effects: Users report temporary improvements in skin appearance, texture, and hydration that satisfy cosmetic goals[19].
- Accessibility: Available through beauty retailers without requiring medical consultation or professional oversight.
Clinical Applications: Medical vs Cosmetic Indications
Optimal Conditions for Mirari Cold Plasma Therapy
Medical cold plasma technology excels in clinical scenarios requiring genuine therapeutic intervention:
- Post-Surgical Care: The dual benefits of antimicrobial action and accelerated healing make cold plasma ideal for surgical site management and complication prevention[20].
- Chronic Skin Conditions: Conditions like diabetic ulcers, pressure sores, and delayed healing wounds benefit from the regenerative capabilities of cold plasma therapy[21].
- Pain Management: The technology’s neural pathway modulation provides alternative pain relief for patients seeking non-pharmaceutical options[22].
When Cosmetic Skincare is Sufficient
Perricone MD products address aesthetic concerns without medical intervention:
- Preventive Anti-Aging: Regular use may help maintain skin appearance and slow visible signs of aging through quality cosmetic ingredients.
- Hydration and Comfort: The formulations provide effective moisturization and temporary plumping effects for cosmetic enhancement.
- Luxury Skincare Experience: Premium packaging and formulation appeal to consumers seeking high-end beauty products.
Treatment Protocols and Application Methods
Mirari Cold Plasma Medical Approach
The Mirari system follows evidence-based medical protocols administered by trained healthcare professionals:
- Professional Assessment: Practitioners evaluate patient conditions, medical history, and treatment goals to develop customized protocols.
- Controlled Environment: Treatments occur in clinical settings with proper safety measures and monitoring equipment.
- Treatment Intervals: Sessions typically last 10-15 minutes with specific intervals between treatments based on condition severity and healing progress[23].
Perricone MD Consumer Application
Cosmetic skincare follows different usage patterns:
- Self-Application: Products are designed for home use without professional supervision or special equipment.
- Daily Routine: Integration into morning and evening skincare regimens with consistent daily application.
- Product Layering: Multiple products in the line can be combined for enhanced cosmetic effects.
Cost Analysis and Value Proposition
Long-term Economic Considerations
- Medical Technology Investment: The Mirari system requires higher initial costs but provides lasting therapeutic benefits through actual tissue healing and regeneration. Healthcare providers report improved patient outcomes that justify the investment[24].
- Cosmetic Product Ongoing Costs: Perricone MD products range from $129 to $300 per item, requiring regular replacement to maintain effects. Annual costs can be substantial when purchasing multiple products in the line[25].
Healthcare vs Beauty Spending
- Medical Approach: Cold plasma therapy addresses underlying tissue conditions, potentially reducing the need for additional medical interventions and providing measurable health improvements.
- Cosmetic Approach: Beauty products provide temporary aesthetic enhancement without addressing underlying skin health or creating lasting changes to tissue quality.
Safety Considerations and Professional Oversight
Medical Device Safety Standards
The Mirari Cold Plasma System meets rigorous medical device standards with comprehensive safety features:
- FDA Validation: Class II medical device clearance ensures safety and efficacy through clinical testing and regulatory review[26].
- Professional Training: Healthcare providers receive specific training on device operation, safety protocols, and treatment optimization.
- Patient Monitoring: Continuous oversight during treatment ensures appropriate response and immediate intervention if needed.
Cosmetic Product Safety
Perricone MD formulations follow cosmetic safety regulations with generally mild ingredient profiles:
- Consumer Testing: Products undergo stability and irritation testing appropriate for cosmetic applications.
- Ingredient Transparency: Complete ingredient lists allow consumers to identify potential allergens or sensitivities.
- Self-Monitoring: Users assess their own skin response and adjust usage as needed.
Future Implications and Technological Evolution
Medical Technology Advancement
The Mirari Doctor platform continues developing enhanced applications and treatment protocols based on clinical feedback and research findings[27]. Future developments may include:
- AI-Enhanced Protocols: Machine learning optimization of treatment parameters based on individual patient responses and tissue characteristics.
- Expanded Clinical Applications: Research continues exploring cold plasma’s potential in treating complex conditions and enhancing therapeutic outcomes.
- Integration with Digital Health: Advanced monitoring capabilities position the technology for integration with electronic health records and telemedicine platforms.
Cosmetic Industry Innovation
Perricone MD continues developing new formulations and delivery systems within the traditional cosmetic framework:
- Enhanced Ingredient Penetration: Improvements to their liquid crystal delivery system may increase cosmetic ingredient efficacy.
- Novel Active Compounds: Research into new anti-aging ingredients and combinations for enhanced cosmetic effects.
- Personalized Skincare: Potential development of customized formulations based on individual skin analysis.
Environmental Impact and Sustainability
Medical Technology Footprint
Cold plasma technology offers environmental advantages through device-based treatment:
- Reduced Chemical Waste: No chemical byproducts or environmental contamination from treatment sessions.
- Durable Equipment: Long-lasting medical devices reduce packaging waste compared to consumable products.
- Energy Efficiency: Modern cold plasma devices operate with minimal power consumption while providing therapeutic effects.
Cosmetic Product Environmental Considerations
Traditional skincare products present different sustainability challenges:
- Packaging Waste: Regular product replacement generates ongoing packaging and container waste.
- Chemical Production: Manufacturing cosmetic ingredients requires various chemical processes and resources.
- Transportation Impact: Global distribution of beauty products contributes to carbon footprint through shipping and retail networks.
Frequently Asked Questions
Is Mirari Cold Plasma more effective than Perricone MD for anti-aging?
Yes, Mirari Cold Plasma provides superior anti-aging results through actual tissue regeneration and collagen synthesis stimulation, while Perricone MD products offer only temporary cosmetic effects. Cold plasma therapy creates lasting cellular changes that improve skin structure and function, whereas cosmetic products require continuous use to maintain appearance benefits[28].
Can I use both technologies together for enhanced results?
The Mirari Doctor platform specifically warns against combining the cold plasma device with other skincare products during treatment, as this may cause unpredictable effects. Healthcare providers recommend using cold plasma therapy as the primary treatment modality, then incorporating appropriate post-treatment skincare as directed by certified medical professionals[29].
Which technology provides better value for anti-aging investment?
Medical cold plasma technology offers superior long-term value despite higher initial costs. While Perricone MD products cost $129–$300 each and require continuous replacement, cold plasma therapy creates permanent tissue improvements that don’t require ongoing product purchases. Healthcare facilities report patient satisfaction with lasting results that justify the medical investment[30].
Are there safety differences between medical and cosmetic approaches?
Medical cold plasma includes comprehensive safety features with FDA validation, professional oversight, and clinical monitoring, while cosmetic products follow basic beauty product safety standards. Cold plasma treatments require healthcare provider supervision to ensure optimal outcomes, whereas cosmetic products rely on consumer self-assessment for safety and effectiveness[31].
How do the scientific mechanisms differ between these technologies?
Mirari Cold Plasma generates genuine ionized gas with reactive species that create therapeutic effects at the cellular level, including nitric oxide production and antimicrobial action. Perricone MD uses no actual plasma technology, instead relying on conventional cosmetic ingredients delivered through their liquid crystal system for surface-level improvements[32].
Conclusion: Medical Innovation vs Marketing Excellence
The comparison between Mirari Cold Plasma and Perricone MD Cold Plasma Plus reveals a fundamental distinction between authentic medical technology and sophisticated cosmetic marketing. While both products target skin improvement, their approaches, mechanisms, and outcomes differ dramatically.
Medical cold plasma technology represents genuine scientific advancement in regenerative medicine. The Mirari system’s FDA clearance validates its therapeutic capabilities through rigorous clinical testing and regulatory oversight. This technology offers lasting tissue improvements through cellular regeneration, antimicrobial action, and healing acceleration that extend far beyond surface-level cosmetic effects.
Perricone MD’s cosmetic line exemplifies excellent skincare formulation and marketing execution. The products contain quality ingredients and provide temporary aesthetic improvements that satisfy many consumers’ beauty goals. However, the “cold plasma” name reflects marketing strategy rather than actual plasma technology.
For people looking for real health benefits, medical cold plasma technology offers better long-term results by improving tissue permanently and being supervised by healthcare professionals. The investment in medical-grade treatment yields lasting benefits that don’t require continuous product replacement or maintenance.
For those prioritizing daily skincare luxury and temporary aesthetic enhancement, Perricone MD products offer quality formulations with immediate cosmetic gratification. The products integrate easily into beauty routines and provide the premium experience many consumers desire.
Healthcare providers increasingly recognize cold plasma technology as a breakthrough in regenerative medicine, offering patients noninvasive alternatives to traditional treatments. The technology’s multi-modal capabilities address multiple conditions simultaneously, improving treatment efficiency and patient outcomes.
Future developments in both categories will likely enhance their respective capabilities. Medical cold plasma technology continues advancing through AI integration and expanded clinical applications, while cosmetic science develops new ingredients and delivery systems for enhanced aesthetic effects.
The choice between medical innovation and cosmetic excellence ultimately depends on individual goals, expectations, and resources. Understanding these distinctions empowers consumers to make informed decisions aligned with their specific needs and desired outcomes.
As the beauty and medical industries continue evolving, the distinction between therapeutic intervention and cosmetic enhancement becomes increasingly important. True cold plasma technology represents the future of regenerative medicine, while quality skincare products like Perricone MD serve essential roles in daily beauty maintenance and consumer satisfaction.
References
- Mirari Doctor. (2024). Handheld Cold Plasma Technology. https://miraridoctor.com/product/
- Perricone MD. (2025). Cold Plasma Plus+ Advanced Serum Concentrate. https://www.perriconemd.com/p/cold-plasma-plus-advanced-serum-concentrate/12932357/
- Mirari Thailand. (2024). Cold Atmospheric Plasma Technology. https://mirari.co.th/en/
- Mirari Doctor. (2024). Cold Plasma: Revolutionary Medical Technology. https://miraridoctor.com/cold-plasma/
- Perricone MD. (2025). Cold Plasma Plus+ Collection Overview. https://www.perriconemd.co.uk/c/collections/cold-plasma-plus/
- General Vibronics Inc. (2024). MIRARI Cold Plasma System Technical Specifications. https://generalvibronics.com
- Mirari Doctor. (2024). Mirari Cold Plasma vs Red Light Therapy. https://miraridoctor.com/mirari-cold-plasma-vs-red-light-therapy/
- Klampfl, T.G., et al. (2012). Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Applied and Environmental Microbiology, 78(15), 5077-5082. https://pubmed.ncbi.nlm.nih.gov/22582068/
- Li, Y.F., et al. (2013). Cold atmospheric plasma changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One, 8(11), e79325. https://doi.org/10.1371/journal.pone.0079325
- Perricone MD. (2025). Cold Plasma Plus+ The Essence. https://www.perriconemd.com/p/cold-plasma-plus-the-essence/14200088/
- General Vibronics Inc. (2024). FDA Clearance Documentation for Mirari Cold Plasma System. Certificate No. 3959-1-2025-1. https://www.fda.gov/medical-devices/recently-approved-devices
- Perricone MD. (2025). Product Safety Information and Ingredients. https://www.perriconemd.com/c/collections/cold-plasma-plus/
- Heinlin, J., et al. (2013). Plasma applications in medicine with a special focus on dermatology. Journal of European Academy of Dermatology and Venereology, 27(1), 1-11. https://doi.org/10.1111/j.1468-3083.2010.03702.x
- Beautiful With Brains. (2020). Is The New Perricone MD Cold Plasma+ Range Worth The Splurge? https://www.beautifulwithbrains.com/perricone-cold-plasma-plus-face-eyes-reviews/
- Schmidt, A., et al. (2017). One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. International Journal of Molecular Sciences, 18(4), 868. https://doi.org/10.3390/ijms18040868
- Brandenburg, R., et al. (2009). Antimicrobial Effects of UV and VUV Radiation of Nonthermal Plasma Jets. IEEE Transactions on Plasma Science, 37(6), 877-883. https://doi.org/10.1109/TPS.2009.2019657
- Mirari Doctor. (2024). Professional Treatment Guidelines and Safety Protocols. https://miraridoctor.com
- Style Sprinter. (2021). Perricone MD Cold Plasma Plus Review. https://stylesprinter.com/perricone-md-cold-plasma-plus-review/
- Sephora UK. (2022). Perricone MD Cold Plasma Plus Face 30ml Customer Reviews. https://www.sephora.co.uk/p/Perricone-MD-Cold-Plasma-Plus-Face-30ml
- Mirari Vietnam. (2022). Post-Surgical Care with US Cold Plasma Technology. https://mirari.vn/cong-nghe-plasma-lanh-hoa-ky-cham-soc-sau-phau-thuat-voi-mirari-beauty/
- Zimmermann, J.L., et al. (2012). Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology, 167(2), 404-410. https://doi.org/10.1111/j.1365-2133.2012.10923.x
- Heinlin, J., et al. (2014). Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clinical Plasma Medicine, 2(2), 50-55. https://doi.org/10.1016/j.cpme.2014.07.001
- Thomas, H.M., et al. (2013). Non-thermal plasma—More than five years of clinical experience. Clinical Plasma Medicine, 1(1), 13-18. https://doi.org/10.1016/j.cpme.2012.11.001
- Karrer, S., et al. (2018). Comparing two different plasma devices regarding their molecular and cellular effects on wound healing. Clinical Plasma Medicine, 9, 24-33. https://doi.org/10.1016/j.cpme.2018.01.002
- Ulta Beauty. (2025). Cold Plasma Plus+ Power Quad Pricing. https://www.ulta.com/p/cold-plasma-plus-power-quad-pimprod2032155?sku=2595061
- FDA. (2024). Medical Device Classification for Cold Plasma Systems. https://www.fda.gov/medical-devices/recently-approved-devices
- Mirari Doctor. (2024). Technology Development and Clinical Applications. https://miraridoctor.com/treatment-guidelines
- Unger, P., et al. (2017). Cold atmospheric plasma activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis. Journal of Dermatological Science, 89(2), 181-190. https://doi.org/10.1016/j.jdermsci.2017.11.008
- Mirari Doctor. (2024). Treatment Protocol Warnings and Guidelines. https://miraridoctor.com/treatment-guidelines
- Isbary, G., et al. (2013). Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds. Clinical Plasma Medicine, 1(2), 25-30. https://doi.org/10.1016/j.cpme.2013.06.001
- Daeschlein, G., et al. (2012). Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. Journal der Deutschen Dermatologischen Gesellschaft, 10(7), 509-515. https://doi.org/10.1111/j.1610-0387.2012.07857.x
- Fridman, G., et al. (2006). Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and Plasma Processing, 26(4), 425-442. https://doi.org/10.1007/s11090-006-9024-4
Related articles
Made in USA