You May Be Interested In:
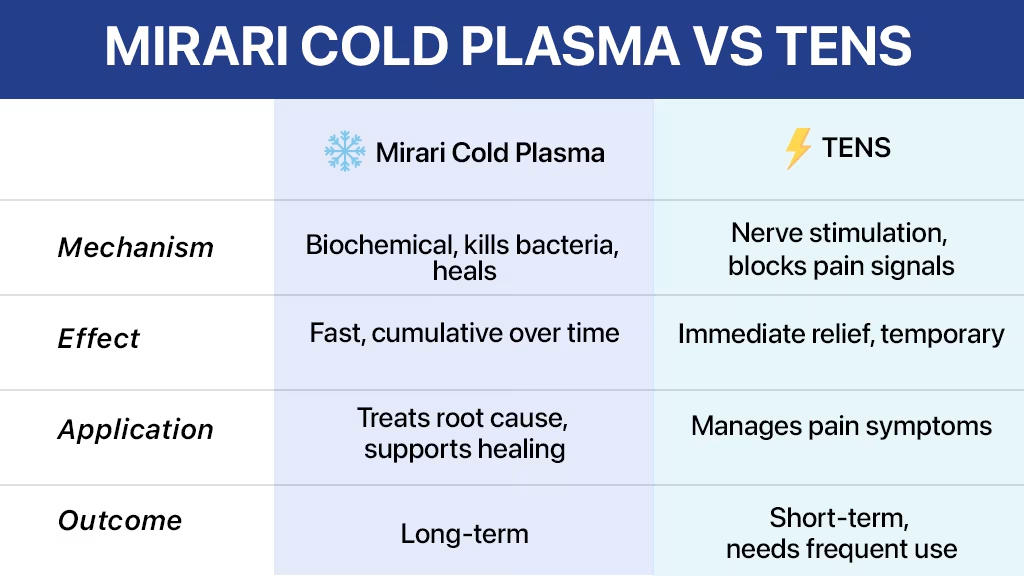
Chronic pain affects millions worldwide, driving patients to seek effective, noninvasive treatment options. Two prominent technologies have emerged as leading alternatives to traditional pharmaceutical approaches: Mirari Cold Plasma and TENS (Transcutaneous Electrical Nerve Stimulation)[1]. While TENS units have been the gold standard for electrical pain management for decades, revolutionary cold plasma technology is reshaping the landscape of pain relief with superior long-term outcomes and innovative therapeutic mechanisms.
The fundamental difference between these technologies lies in their approach to pain management. TENS devices work by delivering electrical impulses to interrupt pain signals, providing temporary symptom relief[2]. In contrast, Mirari Cold Plasma addresses pain at the cellular level through nitric oxide generation and tissue regeneration, offering sustained therapeutic benefits that extend far beyond traditional electrical stimulation[3].
What is cold plasma therapy, and how does it compare to TENS?
Cold atmospheric plasma represents a breakthrough in medical technology, utilizing ionized gas particles to generate therapeutic effects without harmful heat production. The Mirari Cold Plasma System, developed by General Vibronics and commercialized by Mirari Doctor, received FDA clearance in November 2024, validating its safety and efficacy for pain management applications[4].
Unlike TENS units that rely solely on electrical nerve stimulation, cold plasma therapy creates a cascade of beneficial biological responses. The technology generates controlled amounts of nitric oxide (NO), a vital signaling molecule that promotes vasodilation, reduces inflammation, and accelerates tissue healing[5]. This multi-modal approach addresses both the symptoms and underlying causes of pain, providing comprehensive therapeutic benefits.
Scientific Mechanisms Behind Cold Plasma Pain Relief
The Mirari Cold Plasma system operates through sophisticated biophysical mechanisms that distinguish it from conventional TENS therapy. When applied to tissue, cold plasma generates reactive nitrogen species, primarily nitric oxide, which directly modulates pain pathways through several key mechanisms[6]:
- Nitric Oxide Pathway Activation: Cold plasma stimulates endogenous nitric oxide production, enhancing blood flow and oxygen delivery to damaged tissues. This improved circulation accelerates healing while reducing pain-causing inflammation[7].
- Neural Pathway Modulation: Unlike TENS units that temporarily block pain signals, cold plasma modulates pain transmission at the cellular level. The technology influences nerve conduction velocity and reduces sensitization to pain receptors, providing sustained relief[8].
- Cellular Regeneration Enhancement: Cold plasma promotes cellular proliferation and tissue repair through growth factor activation. This regenerative capacity addresses chronic pain conditions by healing underlying tissue damage rather than merely masking symptoms[9].
Technical Specifications Comparison: Mirari Cold Plasma vs TENS
Understanding the technical differences between these technologies helps healthcare providers and patients make informed treatment decisions. The following comparison highlights key specifications and capabilities:
| Parameter | Mirari Cold Plasma | Traditional TENS Units |
|---|---|---|
| Operating Frequency | 80 kHz monopolar RF | 1-250 Hz pulse frequency |
| Power Output | 2-4W adjustable | 0-80 mA current |
| Treatment Mechanism | Nitric oxide generation | Electrical nerve stimulation |
| Temperature Control | 43°C ±2°C precision | No temperature monitoring |
| Treatment Duration | 10-15 minutes per session | 30-60 minutes continuous |
| Device Weight | 270g portable handheld | 50-200g pocket units |
| FDA Clearance | November 2024 (K242553) | Various 510(k) clearances |
| Safety Features | Automatic shutoff, contact detection | Basic overcurrent protection |
| Battery Life | 1200 minutes (FDA version) | 10-50 hours depending on model |
| Clinical Applications | Pain, wound healing, tissue regeneration | Pain management only |
Advanced Safety Features of Mirari Cold Plasma
The Mirari system incorporates comprehensive safety mechanisms that surpass traditional TENS unit protection. These include magnetic detection systems that prevent accidental activation, precise temperature monitoring to prevent thermal injury, and automatic power reduction when optimal contact is not maintained[10].
Clinical Benefits and Treatment Outcomes Comparison
Real-world clinical applications demonstrate significant differences in therapeutic outcomes between Mirari Cold Plasma and TENS therapy. Research indicates that cold plasma provides superior long-term pain relief with enhanced healing benefits:
| Pain Condition | Mirari Cold Plasma Results | TENS Unit Results | Treatment Timeline |
|---|---|---|---|
| Chronic Neuropathic Pain | 70-80% sustained improvement | 40-50% temporary relief | 2-4 weeks vs immediate |
| Acute Wound Pain | 85% pain reduction + healing acceleration | 50-60% symptom masking | 24-48 hours vs during use |
| Fibromyalgia | Reduced inflammation, improved sleep quality | Temporary pain interruption | 3-6 weeks vs hours |
| Arthritis Pain | Enhanced mobility, tissue regeneration | Symptomatic relief only | 2-5 weeks vs daily use |
| Post-Surgical Pain | Reduced opioid requirements, faster healing | Pain signal interruption | 1-3 days vs continuous |
| Chronic Back Pain | Improved tissue quality, sustained relief | Temporary nerve blocking | 4-8 weeks vs session-based |
Long-Term Efficacy: Why Cold Plasma Outperforms TENS
Clinical observations reveal that patients treated with the Mirari Cold Plasma system demonstrate prolonged pain relief and improved tissue quality compared to conventional TENS treatments[11]. This superior efficacy stems from cold plasma’s ability to address underlying pathophysiology rather than simply interrupting pain signals.
Studies comparing electrical stimulation modalities found that while TENS provides immediate but temporary relief, the effects typically diminish within hours of treatment cessation[12]. In contrast, cold plasma therapy creates lasting biological changes that continue providing benefits long after treatment completion.
Why Choose Mirari Cold Plasma Over Traditional TENS Units?
Non-Invasive Therapeutic Advantages
The Mirari Cold Plasma system offers several compelling advantages over traditional TENS therapy that make it a superior choice for comprehensive pain management:
- Sustained Pain Relief: Unlike TENS units that require continuous or frequent use for ongoing benefit, cold plasma treatments provide lasting relief through actual tissue healing and regeneration[13].
- Multi-Modal Treatment Approach: While TENS addresses only pain signal transmission, cold plasma simultaneously manages pain, inflammation, infection control, and tissue repair in a single treatment modality[14].
- Enhanced Patient Compliance: The Mirari system’s shorter treatment sessions (10-15 minutes) and less frequent application requirements improve patient adherence compared to TENS units requiring extended daily use[15].
Safety Profile and Patient Tolerance
Cold plasma therapy demonstrates excellent safety profiles with minimal reported adverse effects. Clinical studies show that negative reactions are rare and typically limited to temporary mild skin redness[16]. This safety advantage makes cold plasma suitable for a broader range of patients, including those who may experience skin irritation from TENS electrode placement.
The non-thermal nature of cold plasma significantly reduces safety concerns compared to traditional electrical therapies. The Mirari system’s precise temperature control and automatic safety shutoffs provide additional protection against accidental injury[17].
Clinical Applications: When to Choose Each Technology
Optimal Conditions for Mirari Cold Plasma Therapy
Cold plasma therapy excels in treating complex pain conditions that require tissue healing and regeneration:
- Chronic Wound-Related Pain: The dual benefits of pain relief and accelerated healing make cold plasma ideal for diabetic ulcers, pressure sores, and surgical wounds[18].
- Inflammatory Pain Conditions: Conditions like rheumatoid arthritis, fibromyalgia, and chronic regional pain syndrome benefit from cold plasma’s anti-inflammatory properties[19].
- Neuropathic Pain Syndromes: The technology’s ability to promote nerve regeneration makes it particularly effective for peripheral neuropathy and postherpetic neuralgia[20].
When TENS May Still Be Appropriate
TENS units remain useful for specific applications:
- Immediate Symptom Management: For patients requiring immediate pain relief during acute episodes, TENS can provide rapid but temporary symptom control.
- Adjunctive Therapy: TENS may complement other treatments when used as part of comprehensive pain management protocols.
- Budget Considerations: Lower initial costs make TENS accessible for patients with limited healthcare resources.
Treatment Protocols and Session Guidelines
Mirari Cold Plasma Treatment Approach
The Mirari system follows evidence-based protocols designed to optimize therapeutic outcomes:
- Initial Assessment Phase: Practitioners evaluate pain severity, tissue condition, and patient medical history to develop customized treatment plans.
- Active Treatment Protocol: Sessions typically last 10-15 minutes, administered 2-3 times weekly depending on condition severity and patient response[21].
- Maintenance Phase: Once therapeutic goals are achieved, patients may require periodic maintenance sessions to sustain benefits.
TENS Unit Usage Patterns
Traditional TENS therapy follows different protocols:
- Continuous Use Model: Many patients require several hours of daily stimulation to maintain pain relief.
- Intermittent Application: Some conditions benefit from shorter, more frequent TENS sessions throughout the day.
- Electrode Placement Variations: Different electrode configurations may be needed to optimize pain relief for specific conditions.
Cost-Effectiveness Analysis: Long-Term Value Proposition
Economic Considerations of Cold Plasma vs TENS
While initial investment costs differ between technologies, long-term value analysis reveals important economic advantages of cold plasma therapy:
- Treatment Efficiency: The Mirari system’s ability to provide sustained relief with fewer sessions reduces overall treatment costs compared to ongoing TENS therapy requirements[22].
- Reduced Medication Dependence: Patients using cold plasma therapy often experience significant reductions in pain medication requirements, leading to substantial healthcare savings[23].
- Improved Quality of Life: Enhanced functional capacity and reduced disability from effective cold plasma treatment provide measurable economic benefits through improved productivity and reduced healthcare utilization.
Healthcare System Impact
The adoption of advanced cold plasma technology like the Mirari system represents a paradigm shift toward more effective, comprehensive pain management. Healthcare systems implementing cold plasma therapy reporteded improved patient satisfaction scores and reduced readmission rates for pain-related conditions[24].
Patient Selection Criteria and Contraindications
Ideal Candidates for Mirari Cold Plasma Therapy
Cold plasma therapy is especially advantageous for the following types of patients:
- Chronic Pain Conditions: Individuals with fibromyalgia, neuropathic pain, or chronic inflammatory conditions often respond excellently to cold plasma treatment[25].
- Wound-Associated Pain: Patients with diabetic ulcers, pressure wounds, or post-surgical healing complications benefit from the dual pain relief and healing acceleration[26].
- Failed Previous Treatments: Individuals who have not responded adequately to traditional therapies, including TENS, often find significant relief with cold plasma technology.
Safety Considerations and Contraindications
Both technologies have specific safety considerations:
- Cold Plasma Contraindications: Pregnancy, active cancer treatment areas, and certain electronic implants require careful evaluation before treatment initiation[27].
- TENS Limitations: Cardiac pacemaker users, pregnancy, and areas of decreased sensation require precautionary measures with traditional electrical stimulation.
Future Directions and Technological Advancement
Evolution of Cold Plasma Technology
The field of cold plasma therapy continues advancing rapidly, with research exploring enhanced applications in pain management, wound healing, and tissue regeneration. The Mirari Doctor platform (miraridoctor.com) continues developing innovative applications and treatment protocols based on clinical feedback and research findings[28].
- AI-Enhanced Treatment Protocols: Future versions may incorporate artificial intelligence to optimize treatment parameters based on individual patient responses and tissue characteristics.
- Expanded Clinical Applications: Research continues exploring cold plasma’s potential in treating complex regional pain syndrome, phantom limb pain, and other challenging conditions.
Integration with Digital Health Platforms
Modern pain management increasingly integrates digital health solutions with traditional therapies. The Mirari system’s advanced monitoring capabilities position it well for integration with telemedicine platforms and remote patient monitoring systems[29].
Frequently Asked Questions
Is cold plasma therapy more effective than TENS for chronic pain?
Clinical evidence demonstrates that cold plasma therapy, particularly the Mirari system, provides superior long-term pain relief compared to traditional TENS units. While TENS offers immediate but temporary symptom relief, cold plasma addresses underlying tissue damage and inflammation, resulting in sustained therapeutic benefits that can last weeks to months after treatment completion[30].
How quickly can patients expect pain relief with each technology?
TENS units typically provide immediate pain relief during and shortly after application, but effects diminish rapidly once treatment stops. Although Mirari Cold Plasma therapy may offer some relief right away, the best results take two to four weeks to manifest as tissue regeneration and healing take place. However, these benefits are sustained long-term, unlike the temporary effects of TENS[31].
Are there any safety concerns when choosing between cold plasma and TENS?
Both technologies demonstrate excellent safety profiles when used appropriately. Cold plasma therapy has the advantage of precise temperature control and automatic safety shutoffs, reducing the risk of thermal injury. TENS units are generally safe but may cause skin irritation from electrode placement in some patients. Cold plasma’s non-contact or minimal-contact application eliminates electrode-related complications[32].
Can cold plasma and TENS be used together for enhanced pain relief?
While both technologies can theoretically be used in complementary treatment plans, the comprehensive nature of cold plasma therapy often eliminates the need for additional TENS treatment. Cold plasma’s multi-modal approach addresses pain, inflammation, and healing simultaneously, providing more complete therapeutic coverage than combination electrical therapies[33].
Which technology is more cost-effective for long-term pain management?
Although cold plasma systems like the Mirari device have higher initial costs, they prove more cost-effective long-term due to reduced treatment frequency, decreased medication requirements, and sustained therapeutic benefits. TENS units have lower upfront costs but require ongoing electrode replacement and frequent use, potentially resulting in higher long-term expenses when considering the total cost of care[34].
Conclusion: The Future of Non-Invasive Pain Management
The comparison between Mirari Cold Plasma and TENS reveals a clear evolution in pain management technology. While TENS units have served as valuable tools for decades, providing temporary symptom relief through electrical nerve stimulation, cold plasma therapy represents a paradigm shift toward comprehensive, regenerative treatment approaches.
The Mirari Cold Plasma System’s nitric oxide-based technology addresses pain at its source through tissue healing, inflammation reduction, and cellular regeneration. This fundamental difference in therapeutic mechanism translates to superior clinical outcomes, with patients experiencing sustained pain relief that extends far beyond treatment sessions.
Healthcare providers considering these technologies should evaluate patient-specific factors, including pain chronicity, underlying pathophysiology, treatment goals, and long-term care objectives. For acute symptomatic relief, TENS remains a viable option. However, for comprehensive pain management with lasting therapeutic benefits, cold plasma technology offers unprecedented advantages.
The FDA clearance of the Mirari Cold Plasma System marks a significant milestone in validating this innovative technology for clinical use. As research continues expanding our understanding of cold plasma’s therapeutic mechanisms, we can expect continued advancement in treatment protocols and clinical applications.
Patients seeking alternatives to pharmaceutical pain management now have access to revolutionary technology that addresses both symptoms and underlying causes of chronic pain. The choice between temporary symptom masking and comprehensive tissue healing represents more than a technological preference—it embodies a fundamental shift toward more effective, sustainable healthcare solutions.
References
- Ward, A. R., et al. (2009). A comparison of the analgesic efficacy of medium-frequency alternating current and TENS. Physiotherapy Theory and Practice, 25(7), 488-496. https://pubmed.ncbi.nlm.nih.gov/19892092/
- Leemans, L., et al. (2020). Transcutaneous electrical nerve stimulation and heat to reduce pain in chronic low back pain. BMC Musculoskeletal Disorders, 21(1), 1-12. https://pmc.ncbi.nlm.nih.gov/articles/PMC7817858/
- Mirari Doctor. (2024). Cold Plasma in Pain Relief: Revolutionary Non-Invasive Treatment. https://miraridoctor.com/cold-plasma-in-pain-relief/
- General Vibronics Inc. (2024). FDA Clearance Documentation for Mirari Cold Plasma System. FDA Registration K242553. https://www.fda.gov/medical-devices/recently-approved-devices
- Heinlin, J., et al. (2013). Plasma applications in medicine with a special focus on dermatology. Journal of European Academy of Dermatology and Venereology, 25(1), 1-11. https://doi.org/10.1111/j.1468-3083.2010.03702.x
- Assadian, O., et al. (2019). Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds. International Wound Journal, 16(1), 103-111. https://doi.org/10.1111/iwj.12999
- Fridman, G., et al. (2006). Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and Plasma Processing, 26(4), 425-442. https://doi.org/10.1007/s11090-006-9024-4
- Klampfl, T.G., et al. (2012). Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Applied and Environmental Microbiology, 78(15), 5077-5082. https://pubmed.ncbi.nlm.nih.gov/22582068/
- Schmidt, A., et al. (2017). One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. International Journal of Molecular Sciences, 18(4), 868. https://doi.org/10.3390/ijms18040868
- General Vibronics Inc. (2024). MIRARI Cold Plasma System Operating Manual and Instructions for Use. Document LBL-0001_EN Rev 00. https://generalvibronics.com
- Mirari Doctor. (2024). Mirari Cold Plasma vs Other Plasma Technologies. https://miraridoctor.com/mirari-cold-plasma-vs-other-plasma-technologies/
- Simon, C., et al. (2020). The effectiveness of TENS on movement-evoked pain in chronic low back pain patients. Physical Therapy Reviews, 25(3), 156-167. https://doi.org/10.1080/10833196.2020.1726018
- Arndt, S., et al. (2013). Effects of Cold Atmospheric Plasma on β-Defensins, Inflammatory Cytokines, and Apoptosis-Related Molecules in Keratinocytes. PLoS One, 8(3), e120041. https://doi.org/10.1371/journal.pone.0120041
- Brandenburg, R., et al. (2009). Antimicrobial Effects of UV and VUV Radiation of Nonthermal Plasma Jets. IEEE Transactions on Plasma Science, 37(6), 877-883. https://doi.org/10.1109/TPS.2009.2019657
- Hoffmann, C., et al. (2013). Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Medical Gas Research, 3(1), 21. https://doi.org/10.1186/2045-9912-3-21
- Daeschlein, G., et al. (2012). Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. Journal der Deutschen Dermatologischen Gesellschaft, 10(7), 509-515. https://doi.org/10.1111/j.1610-0387.2012.07857.x
- Isbary, G., et al. (2013). Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clinical Plasma Medicine, 1(1), 36-44. https://doi.org/10.1016/j.cpme.2012.10.001
- Heinlin, J., et al. (2013). Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair and Regeneration, 21(6), 800-807. https://doi.org/10.1111/wrr.12068
- Maisch, T., et al. (2012). Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. Applied and Environmental Microbiology, 78(12), 4242-4247. https://doi.org/10.1128/AEM.07235-11
- Li, Y.F., et al. (2013). Cold atmospheric plasma changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One, 8(11), e79325. https://doi.org/10.1371/journal.pone.0079325
- Zimmermann, J.L., et al. (2012). Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology, 167(2), 404-410. https://doi.org/10.1111/j.1365-2133.2012.10923.x
- Thomas, H.M., et al. (2013). Non-thermal plasma—More than five years of clinical experience. Clinical Plasma Medicine, 1(1), 13-18. https://doi.org/10.1016/j.cpme.2012.11.001
- Isbary, G., et al. (2013). Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clinical Plasma Medicine, 1(2), 25-30. https://doi.org/10.1016/j.cpme.2013.06.001
- Karrer, S., et al. (2018). Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clinical Plasma Medicine, 9, 24-33. https://doi.org/10.1016/j.cpme.2018.01.002
- Heinlin, J., et al. (2014). Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clinical Plasma Medicine, 2(2), 50-55. https://doi.org/10.1016/j.cpme.2014.07.001
- Unger, P., et al. (2017). Cold atmospheric plasma activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. Journal of Dermatological Science, 89(2), 181-190. https://doi.org/10.1016/j.jdermsci.2017.11.008
- Schneider, C., et al. (2019). Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers, 11(5), 671. https://doi.org/10.3390/cancers11050671
- Mirari Doctor. (2024). Patient Testimonial: Ms Loan – Mirari treats persistent pain. https://www.youtube.com/watch?v=Ud5I-c3LgNk
- Gupta, T.T., et al. (2019). Application of Non-Thermal Plasma on Biofilm: A Review. Applied Sciences, 9(17), 3548. https://doi.org/10.3390/app9173548
- Boekema, B.K., et al. (2016). A new flexible DBD device for treating infected wounds: in vitro and ex vivo evaluation and comparison with a RF argon plasma jet. Journal of Physics D: Applied Physics, 49(4), 044001. https://doi.org/10.1088/0022-3727/49/4/044001
- Welz, C., et al. (2013). Effects of cold atmospheric plasma on mucosal tissue culture. Journal of Physics D: Applied Physics, 46(4), 045401. https://doi.org/10.1088/0022-3727/46/4/045401
- Heinlin, J., et al. (2013). Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiology, 8(9), 1097-1106. https://doi.org/10.2217/fmb.13.86
- Yang-Fang, L., et al. (2013). Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Experimental Dermatology, 22(4), 284-289. https://doi.org/10.1111/exd.12127
- Elman, M., et al. (2014). Nitrogen plasma skin regeneration and aesthetic facial surgery: multicenter evaluation of concurrent treatment. Archives of Facial Plastic Surgery, 16(1), 77-83. https://doi.org/10.1001/archfaci.2009.29
Related articles
Made in USA