Mirari Driver

Mirari Magnetic Connector Cable (x2)
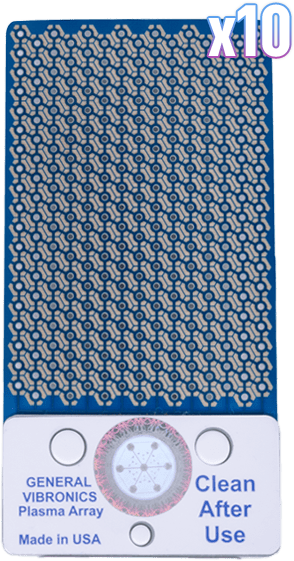
Mirari Plasma Array (x10)

Mirari Retail Bundle

MirariCare+
Mirari Cold Plasma System Disclaimer
Purpose: MIRARI® Cold Plasma System is a Class II medical device designed exclusively for use by trained healthcare professionals. It is registered and approved for use in Thailand, Vietnam and FDA-cleared in the United States. The device’s usage is intended to comply with applicable regulations, specific intended-for-use and guidelines within these jurisdictions
Informational Use: The MIRARI® Cold Plasma System offers selected operational modes and modulated frequency settings associated with a range of pain levels (from 1 to 10) for educational training and informational purposes only. Users should refer to the product’s Operating Manual for guidance and consult with a qualified healthcare provider for professional medical advice.
Variable Outcomes: The use of selected modes and frequencies may produce varying results based on individual patient conditions and clinical applications. The manufacturer and distributor make no claims or guarantees regarding specific medical outcomes or efficacy
Consultation: Healthcare providers are advised to use the Mirari Cold Plasma System in accordance with their professional judgment and applicable medical protocols. Patients and users should always consult a healthcare professional before relying on the device for treatment.
Liability: By using the MIRARI® Cold Plasma System, users acknowledge and accept all potential risks associated with its operation. The manufacturer and distributor disclaim any liability for adverse effects, injuries, or damages resulting from the use or misuse of the device or its operational settings.
Geographical Availability: The MIRARI® Cold Plasma System is FDA-cleared in the United States and approved by the Thai FDA and Vietnam MOH. Usage outside these approved regions is subject to local regulatory compliance.
User Responsibilit: The selection of operational modes and modulated frequencies is provided as a guide and does not substitute for clinical judgment. Users operate the device at their own risk and are solely responsible for ensuring adherence to safe and proper usage protocols