Is cold plasma safe for medical use? This question has become increasingly important as cold atmospheric plasma (CAP) technology gains recognition in healthcare settings worldwide. Based on extensive clinical research and safety studies, cold plasma therapy demonstrates an excellent safety profile when properly administered by trained healthcare professionals, making it a promising alternative to traditional thermal treatments.
Cold plasma technology operates at room temperature while generating therapeutic reactive species, eliminating the thermal damage risks associated with conventional plasma treatments. Understanding the safety considerations, potential side effects, and proper application protocols is essential for both healthcare providers and patients considering this innovative therapy.
Understanding Cold Plasma Safety Fundamentals
What Makes Cold Plasma Safe for Medical Applications?
Cold atmospheric plasma represents a significant advancement in medical technology due to its unique operational characteristics that prioritize patient safety. Unlike thermal plasma that operates at extreme temperatures, cold plasma maintains temperatures close to body temperature while delivering therapeutic benefits[1].
The safety profile of cold plasma stems from its controlled energy delivery system. The technology generates reactive oxygen and nitrogen species at physiological temperatures, typically between 20-40°C, which eliminates the risk of thermal burns or tissue damage commonly associated with traditional thermal therapies.
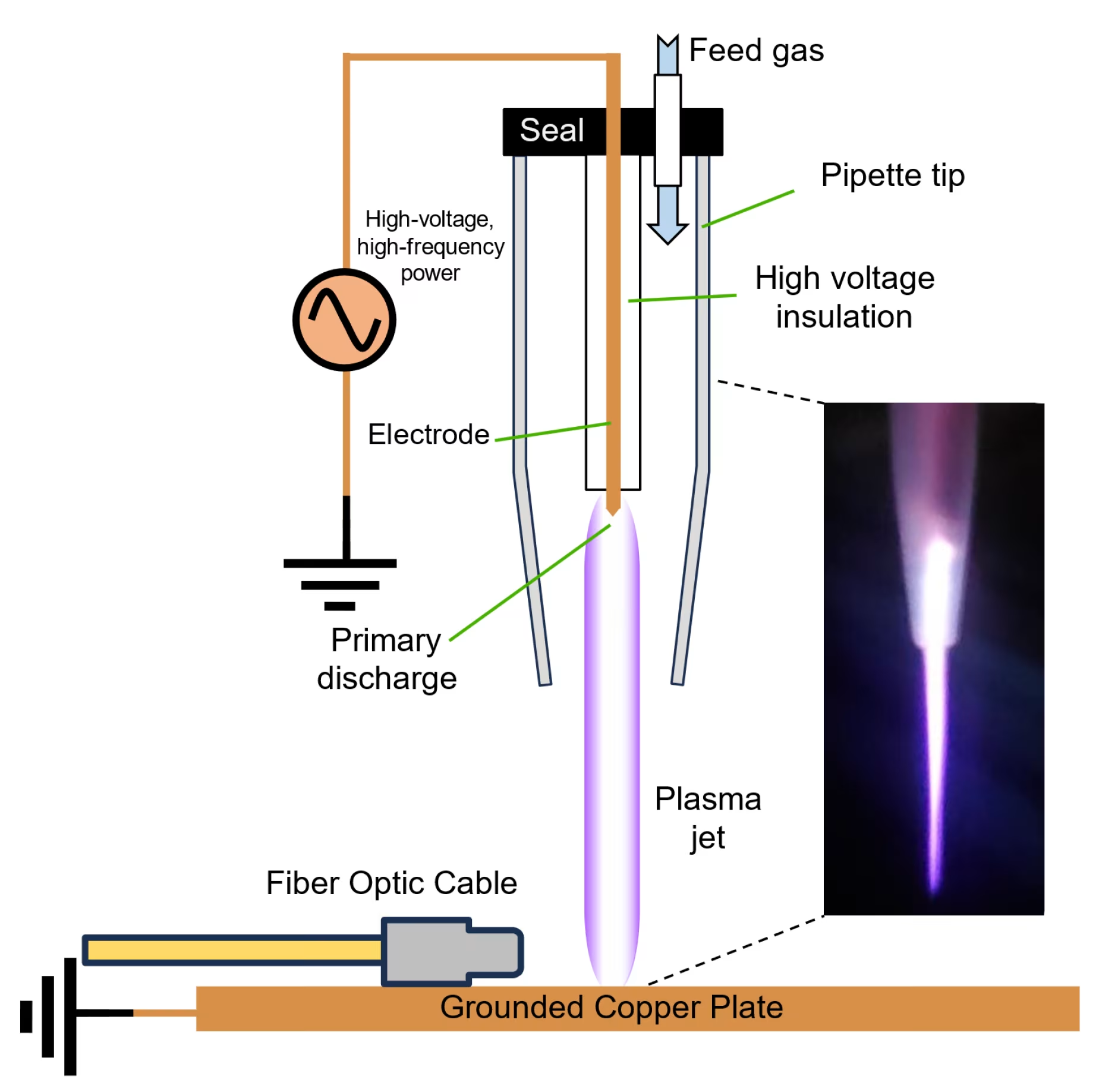
Modern cold plasma devices, including the Mirari Cold Plasma System developed by General Vibronics, incorporate advanced safety features such as automatic temperature monitoring, treatment duration controls, and real-time feedback systems to ensure patient protection throughout the treatment process.
Scientific Evidence Supporting Cold Plasma Safety
Extensive clinical research has consistently demonstrated the safety of cold plasma therapy across various medical applications. A comprehensive study involving 298 cold plasma treatments found that 56.9% of patients experienced no pain during treatment, while 40.1% reported only mild discomfort[2].
Long-term safety studies have shown no malignant changes, inflammatory reactions, or pathological changes in cell architecture following cold plasma treatment[3]. These findings provide strong evidence that cold plasma therapy, when properly administered, does not cause lasting harm to healthy tissues.
Research has also confirmed that cold plasma does not affect skin moisture, cause irritation, or alter the stratum corneum or mucous membrane when used according to established protocols[2].
Technical Safety Specifications and Controls
| Safety Parameter | Specification | Clinical Significance |
|---|---|---|
| Operating Temperature | 20-40°C (room temperature) | Eliminates thermal burn risk |
| Treatment Duration | Maximum 15 minutes per session | Prevents overexposure |
| Power Output | 2-4W adjustable | Customizable for patient tolerance |
| Frequency Range | 80 kHz monopolar RF | Optimized for safety and efficacy |
| Safety Shutoff | Automatic temperature monitoring | Prevents thermal damage |
| Session Intervals | Minimum 30 minutes between treatments | Allows tissue recovery |
Common Side Effects and Safety Profile
Mild and Temporary Side Effects
Cold plasma therapy is associated with minimal side effects that are typically mild and temporary. The most commonly reported side effects include slight skin redness, mild discomfort during treatment, and temporary sensitivity at the application site[4].
Clinical studies have documented that these side effects are generally well-tolerated and resolve quickly after treatment completion. Most patients experience no lasting discomfort, and serious adverse events are extremely rare when treatments are performed according to established protocols.
Pain and Discomfort Management
Research involving burn patients showed significant improvements in pain management with cold plasma therapy. Pain levels decreased from 28.4% during treatment to only 7.5% after treatment completion[2]. This demonstrates that any discomfort experienced during treatment is typically brief and well-managed.
Healthcare providers can minimize patient discomfort by:
- Adjusting treatment parameters based on individual tolerance
- Using appropriate cooling measures when necessary
- Providing clear patient education about expected sensations
- Monitoring patient response throughout the treatment
Allergic Reactions and Contraindications
While allergic reactions to cold plasma therapy are rare, they can occur in sensitive individuals. These reactions typically manifest as localized swelling, mild itching, or skin irritation. The Mirari Cold Plasma System’s nitric oxide-based technology has demonstrated a lower incidence of allergic reactions compared to traditional reactive oxygen species (ROS) based systems[4].
Contraindications for cold plasma therapy include:
- Pregnancy and breastfeeding
- Active skin infections at the treatment site
- Certain medical implants near the treatment area
- Compromised immune systems
- Severe cardiovascular conditions
Clinical Safety Evidence and Research Findings
| Application Area | Safety Outcomes | Study Duration | Adverse Events |
|---|---|---|---|
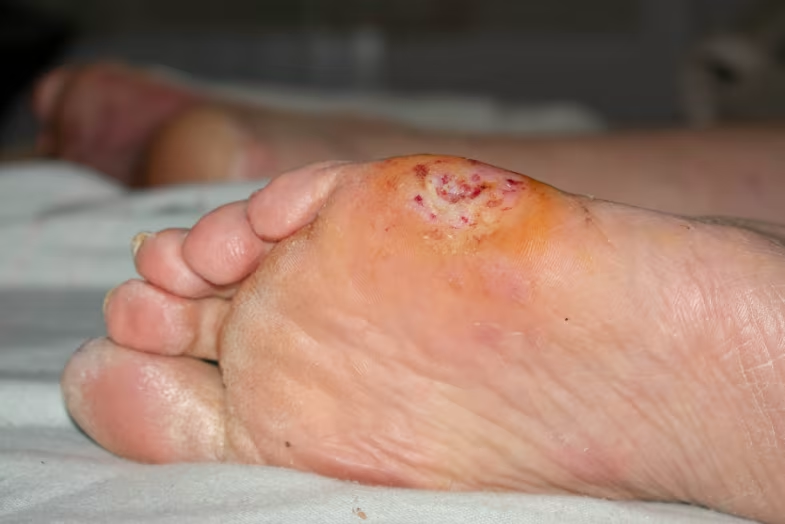
| Diabetic Foot Ulcers | No serious adverse device effects | 10 days treatment | 55% transient mild effects |
| Burn Treatment | Safe and effective with minimal effects | Multiple sessions | Mild pain in 40.1% of cases |
| Atopic Dermatitis | No safety issues reported | 4-week follow-up | Well-tolerated treatment |
| Wound Healing | Excellent safety profile | Long-term studies | Minimal temporary irritation |
| Oral Applications | Well-tolerated with proper protocols | Various durations | Mild local effects only |
Long-Term Safety Considerations
Long-term safety studies have provided reassuring evidence about the sustained safety of cold plasma therapy. A one-year follow-up study showed no detectable long-term side effects in experimental models after consecutive treatments[4].
Clinical observations in humans over extended periods have reported minimal long-term impacts, with only occasional instances of mild local reactions that resolved without intervention. These findings support the use of cold plasma therapy for repeated treatments when medically indicated.
Safety in Special Populations
Cold plasma therapy has been studied in various patient populations with encouraging safety results. Research in diabetic patients with foot ulcers showed that cold atmospheric plasma treatment was safe and well-tolerated, with ulcer size and bacterial colonization decreasing during treatment[5].
Studies in patients with atopic dermatitis demonstrated that cold plasma can significantly improve mild and moderate symptoms without safety issues[6]. These findings indicate that cold plasma therapy can be safely used across different patient populations when appropriate protocols are followed.
Safety Protocols and Best Practices
Healthcare Provider Training and Certification
Ensuring the safety of cold plasma therapy requires proper training and certification of healthcare providers. The Mirari Cold Plasma System, available through miraridoctor.com, emphasizes the importance of comprehensive training programs for healthcare professionals to ensure safe and effective treatment delivery.
Key training components include:
- Understanding device operation and safety features
- Proper patient assessment and selection
- Treatment parameter adjustment based on patient needs
- Recognition and management of adverse reactions
- Emergency response protocols
Patient Assessment and Preparation
Thorough patient assessment is crucial for ensuring cold plasma therapy safety. Healthcare providers should conduct comprehensive evaluations including:
Medical history review to identify contraindications Skin condition assessment at the treatment site Allergy screening for potential sensitivities Discussion of patient expectations and concerns Informed consent process with clear explanation of risks and benefits
Treatment Monitoring and Documentation
Continuous monitoring during cold plasma treatment ensures immediate detection and response to any adverse reactions. Healthcare providers should maintain detailed documentation of:
- Treatment parameters used
- Patient response and tolerance
- Any side effects or complications
- Treatment outcomes and follow-up care
Comparing Cold Plasma Safety to Alternative Treatments
Safety Advantages Over Traditional Therapies
Cold plasma therapy offers significant safety advantages compared to many conventional treatments. Unlike laser therapy that can cause thermal damage, chemical treatments that may have systemic effects, or surgical interventions with infection risks, cold plasma provides therapeutic benefits with minimal adverse effects.
The non-thermal nature of cold plasma eliminates many risks associated with heat-based treatments while providing comparable or superior therapeutic outcomes. This safety profile makes cold plasma particularly attractive for patients who may not be candidates for more aggressive interventions.
Risk-Benefit Analysis
When evaluating the safety of cold plasma therapy, the risk-benefit ratio is highly favorable. The minimal side effects and excellent safety profile, combined with demonstrated therapeutic efficacy, make cold plasma an attractive treatment option for various medical conditions.
Healthcare providers can confidently recommend cold plasma therapy for appropriate patients, knowing that the treatment carries minimal risk while offering significant potential benefits for wound healing, pain management, and tissue regeneration.
Future Safety Research and Development
Ongoing Safety Studies
Continued research into cold plasma safety focuses on optimizing treatment protocols, expanding applications, and further characterizing long-term effects. Current investigations include studies on optimal treatment parameters, combination therapies, and applications in new medical specialties.
The Mirari Cold Plasma System continues to contribute to this research through clinical studies and post-market surveillance, helping to refine safety protocols and expand the understanding of cold plasma therapy’s safety profile.
Regulatory Oversight and Standards
Cold plasma devices are subject to rigorous regulatory oversight to ensure patient safety. The FDA clearance of devices like the Mirari Cold Plasma System demonstrates compliance with stringent safety and efficacy standards.
Ongoing regulatory monitoring and post-market surveillance help maintain high safety standards while supporting continued innovation in cold plasma technology.
Patient-Focused Safety Questions
Is cold plasma therapy safe for all skin types?
Cold plasma therapy is generally safe for all skin types when proper protocols are followed. However, individuals with very sensitive skin may require modified treatment parameters or additional precautions. Healthcare providers assess each patient individually to determine the most appropriate treatment approach. The Mirari Cold Plasma System’s adjustable settings allow for customization based on skin sensitivity and patient tolerance.
What should I expect during my first cold plasma treatment?
During your first cold plasma treatment, you may experience mild tingling or warmth at the treatment site. Most patients describe the sensation as comfortable and well-tolerated. Your healthcare provider will monitor your response throughout the treatment and can adjust parameters if needed. Any mild redness or sensitivity typically resolves within a few hours after treatment completion.
Are there any long-term safety concerns with repeated cold plasma treatments?
Current research shows no significant long-term safety concerns with repeated cold plasma treatments when administered according to established protocols. Long-term studies have found no evidence of tissue damage, cellular changes, or other adverse effects from multiple treatment sessions. Your healthcare provider will develop an appropriate treatment schedule based on your specific condition and response to therapy.
Can cold plasma therapy interact with my medications?
Cold plasma therapy is a localized treatment that does not typically interact with systemic medications. However, you should inform your healthcare provider about all medications you’re taking, including topical treatments, as some may affect skin sensitivity or healing response. Your provider will assess any potential interactions and adjust your treatment plan accordingly.
What should I do if I experience side effects after cold plasma treatment?
If you experience any side effects after cold plasma treatment, contact your healthcare provider promptly. Most side effects are mild and temporary, but your provider can assess your symptoms and provide appropriate guidance. Common mild effects like slight redness or sensitivity typically resolve on their own, but persistent or concerning symptoms should be evaluated by your healthcare team.
Medical Disclaimer: This information is for educational purposes only and should not replace professional medical advice. Always consult with qualified healthcare providers before starting any new treatment. Individual responses to cold plasma therapy may vary, and treatment decisions should be based on comprehensive medical evaluation and professional judgment.
References
- Braný, D., et al. (2020). Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. International Journal of Molecular Sciences. https://pmc.ncbi.nlm.nih.gov/articles/PMC7215620/
- Vietnam Medical Journal. (2023). Investigating Adverse Effects of Cold Plasma in Treating Second and Third Degree Burns. https://tapchiyhocvietnam.vn/index.php/vmj/article/download/5055/4625/9322
- PMC. (2020). Long-Term Risk Assessment for Medical Application of Cold Atmospheric Plasma. https://pmc.ncbi.nlm.nih.gov/articles/PMC7235715/
- Mirari Doctor. (2024). Cold Plasma Treatment Side Effects: What You Need to Know. https://miraridoctor.com/cold-plasma-treatment-side-effects/
- Lagrand, R.S., et al. (2023). Cold plasma treatment is safe for diabetic foot ulcers and reduces bacterial load. PubMed. https://pubmed.ncbi.nlm.nih.gov/37029969/
- Nature. (2021). Prospective, comparative clinical pilot study of cold atmospheric plasma treatment. https://www.nature.com/articles/s41598-021-93941-y
Related articles
Made in USA