You May Be Interested In:
- Cold Plasma for Pressure Ulcers: Revolutionary Treatment for Non-Healing Wounds
- How to Heal My Wound Faster? Revolutionary Methods and Advanced Technology
- How to Increase Wound Healing? Advanced Strategies for Faster Recovery
- What is a Diabetic Foot Ulcer? Complete Guide to Prevention, Treatment, and Life-Saving Care
- What to Do When Wound is Not Healing? Expert Guide
Wound healing represents one of healthcare’s most complex challenges, affecting millions of patients worldwide who struggle with chronic wounds, surgical complications, and delayed recovery. Modern wound healing approaches have evolved beyond traditional dressings to embrace innovative technologies that accelerate tissue repair, reduce infection rates, and improve patient outcomes. Cold atmospheric plasma (CAP) therapy has emerged as a groundbreaking solution in wound healing, offering non-invasive treatment that harnesses reactive oxygen and nitrogen species to promote faster, more effective tissue regeneration.
Understanding the Science of Wound Healing
Wound healing occurs through four distinct phases: hemostasis, inflammation, proliferation, and remodeling. Each phase requires specific cellular activities and molecular signals to progress effectively[1]. When any phase becomes disrupted or prolonged, wounds can become chronic, leading to significant patient suffering and healthcare costs.
The Four Phases of Wound Healing
- Hemostasisbegins immediately after injury, lasting up to two days. Blood vessels constrict to reduce bleeding while clotting factors create fibrin clots that seal damaged vessels[1]. This initial response sets the foundation for subsequent healing phases.
- Inflammationfollows hemostasis, involving white blood cells and enzymes that clear bacteria and debris while preparing the wound bed for new tissue growth[1]. Physical characteristics include redness, swelling, heat, and pain as the immune system responds to injury.
- Proliferationfocuses on filling and covering the wound through granulation tissue formation, angiogenesis, wound contraction, and epithelialization[1]. This phase determines the quality and speed of tissue repair.
- Remodelingrepresents the final phase where collagen reorganizes to strengthen the healed tissue, potentially lasting months to years depending on wound severity and patient factors.
Factors Affecting Wound Healing
Multiple factors influence wound healing success, including patient age, nutrition status, underlying medical conditions, infection presence, and treatment approaches. Chronic conditions like diabetes significantly impair wound healing by affecting circulation, immune function, and cellular metabolism[2].
Revolutionary Cold Plasma Technology in Wound Healing
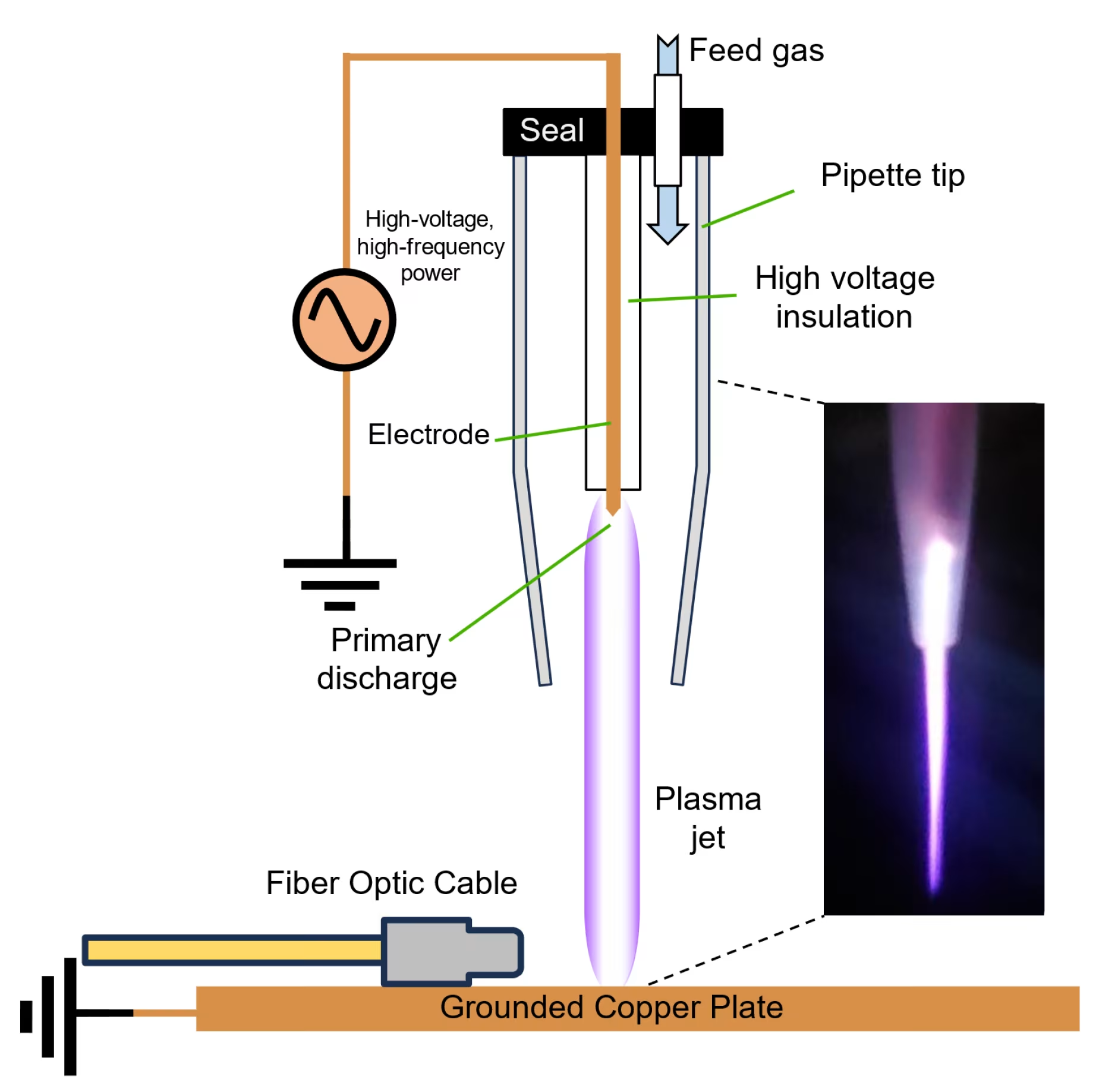
Cold atmospheric plasma represents a breakthrough in wound healing technology, utilizing ionized gases at room temperature to generate therapeutic reactive species without causing thermal damage[3]. This innovative approach addresses multiple wound healing challenges simultaneously through antimicrobial effects, cellular stimulation, and inflammation modulation.
Scientific Mechanisms Behind Cold Plasma Wound Healing
Cold plasma generates a complex mixture of reactive oxygen and nitrogen species (RONS) that interact with wound tissues through multiple pathways[4]. These species include hydroxyl radicals, hydrogen peroxide, nitric oxide, and superoxide anions that work synergistically to promote healing.
The Mirari Cold Plasma system, developed by General Vibronics and available through Mirari Doctor (miraridoctor.com), represents a significant advancement in cold plasma wound healing technology. This device utilizes nitric oxide-enriched plasma delivery, offering enhanced precision in targeting specific wound healing pathways[5].
Nitric Oxide vs Reactive Oxygen Species in Wound Healing
Traditional cold plasma devices primarily generate reactive oxygen species through atmospheric ionization. However, the Mirari Cold Plasma system utilizes nitric oxide (NO) as the primary therapeutic agent, providing enhanced selectivity for wound healing applications[5]. Nitric oxide plays crucial roles in wound healing by promoting vasodilation, enhancing nutrient delivery, and supporting natural repair mechanisms.
Antimicrobial Effects in Wound Healing
Cold plasma demonstrates broad-spectrum antimicrobial activity against bacteria, viruses, and fungi, including antibiotic-resistant strains like MRSA[6]. This antimicrobial effect occurs through multiple mechanisms including membrane disruption, protein denaturation, and DNA damage in pathogenic microorganisms.
Clinical studies show significant bacterial load reduction following cold plasma treatment, helping manage persistent infections that plague chronic wounds[7]. Unlike antibiotics, cold plasma’s multifactorial action reduces the likelihood of resistance development.
Technical Specifications for Wound Healing Applications
| Parameter | Specification | Wound Healing Application |
|---|---|---|
| Operating Temperature | 26-43°C | Safe for direct wound contact without thermal damage |
| Treatment Duration | 30 seconds – 15 minutes | Customizable based on wound type and severity |
| Plasma Generation | Dielectric Barrier Discharge | Stable, controlled plasma formation over wound areas |
| Penetration Depth | 3-8 mm | Targets multiple tissue layers for comprehensive healing |
| Gas Composition | Atmospheric air/specialized mixtures | No external gas requirements for most applications |
| Power Output | Variable intensity | Adjustable for different wound healing needs |
Clinical Applications of Cold Plasma in Wound Healing
Acute Wound Healing Applications
Cold plasma technology demonstrates exceptional efficacy in acute wound healing scenarios where rapid tissue repair is essential. In surgical wound applications, cold plasma accelerates natural healing processes through multiple simultaneous mechanisms[8].
Clinical evidence shows patients with post-surgical wounds treated with cold plasma demonstrated 50% reduction in wound size within one week compared to standard dressings alone[5]. This accelerated healing translates to reduced hospital stays, lower infection rates, and improved patient satisfaction.
Benefits for Acute Wound Management
Reduced bacterial colonizationprevents post-surgical infections whileenhanced tissue regenerationoccurs through growth factor stimulation.Decreased inflammationpromotes faster recovery, andimproved wound closure ratesreduce overall healing time.Minimized scarringresults from controlled tissue repair processes.
Chronic Wound Healing Management
Chronic wound healing represents one of the most challenging applications in healthcare, with traditional treatments often failing to achieve satisfactory outcomes. Cold plasma addresses these complex cases through its multi-modal approach to wound healing[9].
A randomized controlled trial involving 43 patients with diabetic foot ulcers demonstrated that cold plasma therapy led to significant wound area reduction and accelerated wound closure compared to standard care[10]. The study showed meaningful improvements in wound healing parameters without treatment-related adverse events.
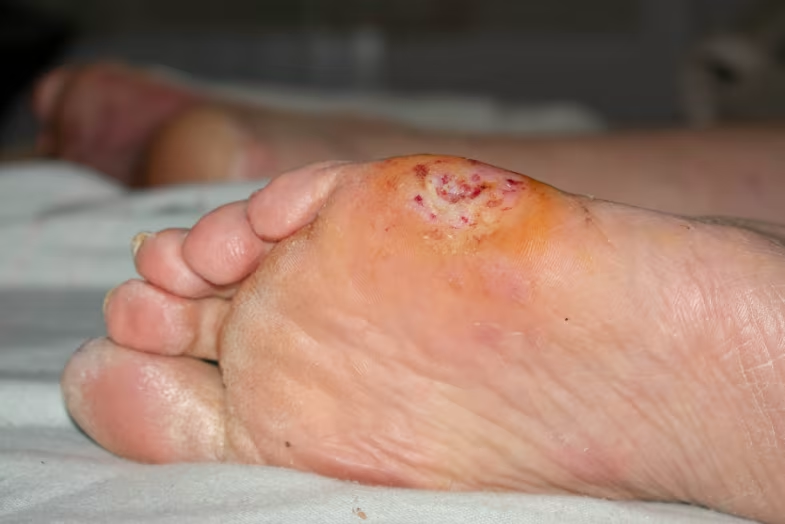
Specialized Chronic Wound Applications
Diabetic foot ulcersbenefit from cold plasma’s ability to improve oxygen supply and nutrient delivery to damaged tissue layers[10].Venous leg ulcersshow improved closure rates with cold plasma treatment.Pressure ulcersrespond well to the therapy’s antimicrobial and tissue regeneration properties.
Clinical Benefits and Treatment Outcomes
| Wound Type | Treatment Protocol | Clinical Outcomes | Evidence Level |
|---|---|---|---|
| Diabetic Foot Ulcers | 3x/week for 3 weeks | Significant wound size reduction, improved healing rates | Randomized controlled trial |
| Chronic Ulcers | Weekly treatments for 4 weeks | 28% average wound size reduction, improved granulation | Multicenter observational study |
| Surgical Wounds | As needed, individualized | 50% size reduction in one week, reduced infection | Case studies |
| Burn Wounds | Daily treatments, 2-4 weeks | Enhanced re-epithelialization, reduced pain | Clinical trials |
| Infected Wounds | 2-3x/week until resolved | Bacterial load reduction, accelerated closure | Clinical observation |
| Pressure Ulcers | 3x/week maintenance | Enhanced granulation, improved tissue quality | Case series |
Long-term Wound Healing Outcomes
Follow-up studies demonstrate sustained wound healing benefits extending beyond active treatment periods. The comprehensive analysis revealed that wound healing improvements continued for 25 weeks post-treatment, indicating lasting therapeutic effects[11].
Long-term benefits include sustained wound closure with low recurrence rates, improved tissue quality with enhanced structural integrity, and reduced infection rates during healing periods. Patients also experience enhanced quality of life through pain reduction and lower healthcare utilization due to improved outcomes.
Comparing Cold Plasma to Traditional Wound Healing Methods
Advantages Over Conventional Wound Care
Cold plasma offers significant advantages over traditional wound healing approaches through its comprehensive therapeutic mechanisms. Traditional wound care often relies on passive strategies like moisture management and barrier protection, while cold plasma actively promotes healing through biological stimulation[12].
Key advantages include immediate antimicrobial effects versus delayed antibiotic action, multi-pathway healing stimulation compared to single-mechanism treatments, and no resistance development unlike antibiotic-based wound care. Cold plasma provides simultaneous infection control and tissue regeneration with reduced treatment duration compared to conventional methods.
Cold Plasma vs Advanced Wound Healing Technologies
Compared to other advanced wound healing technologies, cold plasma provides unique advantages through its non-invasive nature and comprehensive therapeutic effects. Unlike hyperbaric oxygen therapy requiring specialized facilities or negative pressure therapy requiring complex equipment management, cold plasma offers portable, immediate wound healing intervention[13].
Comparative advantages include lower cost per treatment than facility-based therapies, immediate availability without scheduling constraints, no consumable supplies required beyond device maintenance, and suitability for all wound types without contraindications related to wound characteristics.
Safety Considerations in Wound Healing Applications
Clinical Safety Profile
Cold plasma demonstrates excellent safety profiles in wound healing applications, with clinical studies reporting minimal adverse effects[14]. In burn treatment studies, 71.6% of patients experienced only mild pain during treatment, decreasing to 27.2% post-treatment.
Common side effects include temporary mild redness, slight tingling sensations, and minor skin irritation that typically resolves within hours. No serious adverse events have been reported in clinical trials, making cold plasma suitable for diverse patient populations.
Treatment Guidelines and Precautions
Appropriate wound healing applications include chronic wounds unresponsive to conventional treatment, infected wounds requiring antimicrobial intervention, post-surgical wounds requiring enhanced healing, and diabetic ulcers with complex healing challenges[15].
Contraindications include active malignancy in the wound area and severe bleeding disorders. Relative contraindications include pregnancy and implanted devices near treatment sites. Healthcare providers should evaluate each wound individually to determine treatment appropriateness.
Implementation in Clinical Practice
Integrating Cold Plasma into Wound Care Protocols
The integration of cold plasma technology into existing wound healing protocols facilitates seamless adoption without disrupting established workflows. The handheld nature of devices like the Mirari Cold Plasma system requires minimal training and can be incorporated into routine wound care procedures[5].
Integration benefits include point-of-care treatment eliminating referral delays, enhanced wound care capabilities without facility modifications, and improved patient satisfaction through advanced treatment options. Healthcare systems report significant cost savings through reduced wound care duration and improved healing success rates.
Economic Impact of Enhanced Wound Healing
The economic advantages of improved wound healing extend beyond device costs to include reduced hospitalization, fewer complications, and enhanced patient productivity. Financial benefits include reduced wound care supply costs through faster healing, decreased antibiotic usage lowering pharmaceutical expenses, and shorter treatment duration improving resource utilization[16].
Future Directions in Wound Healing Technology
Emerging Applications and Research
Research continues to expand cold plasma applications in wound healing, with particular focus on combination therapies and personalized treatment protocols. Studies show that combining cold plasma with conventional treatments achieves synergistic effects, often improving outcomes while reducing overall treatment complexity.
The development of standardized treatment protocols and regulatory frameworks will be crucial for widespread clinical adoption. Current efforts focus on establishing consistent parameters for different wound healing applications and optimizing treatment schedules based on wound characteristics and patient responses.
Innovation in Device Technology
Advances in cold plasma device design continue to improve treatment precision and patient comfort. The Mirari Cold Plasma system exemplifies these innovations through its nitric oxide-enriched plasma technology and user-friendly design that makes advanced wound healing accessible in various clinical settings.
Frequently Asked Questions
How quickly can patients expect to see wound healing improvements with cold plasma therapy?
Clinical studies demonstrate that wound healing improvements can be observed as early as day 2 of treatment, with continued progress throughout the treatment course. A multicenter study showed that improved wound closure was observable within 48 hours and continued for 25 weeks. Case studies report 50% reduction in wound size within one week for post-surgical wounds treated with cold plasma compared to standard dressings.
Is cold plasma therapy safe for all types of wounds?
Cold plasma demonstrates excellent safety profiles across various wound types, with clinical studies reporting minimal adverse effects. The therapy is suitable for acute wounds, chronic wounds, infected wounds, and burns. However, contraindications include active malignancy in the wound area and severe bleeding disorders. Healthcare providers should evaluate each wound individually to determine treatment appropriateness.
What are the side effects of cold plasma wound healing treatment?
Clinical studies show that cold plasma wound healing treatment has minimal side effects. In burn treatment studies, 71.6% of patients experienced mild pain during treatment, which decreased to 27.2% post-treatment. Common side effects include temporary mild redness, slight tingling sensations, and minor skin irritation that typically resolves within hours. No serious adverse events have been reported in clinical trials.
How does cold plasma wound healing compare to traditional wound care methods?
Cold plasma wound healing offers significant advantages over traditional methods through its multi-modal therapeutic approach. Studies demonstrate superior wound closure rates compared to standard wound therapy. Additionally, cold plasma-treated patients required significantly less antibiotic therapy (4%) compared to standard care (23%). Unlike passive wound care strategies, cold plasma actively promotes healing through antimicrobial effects, cellular stimulation, and angiogenesis enhancement.
Can cold plasma be used alongside other wound healing treatments?
Yes, cold plasma shows excellent compatibility with conventional wound healing therapies. Clinical studies demonstrate enhanced efficacy when combined with standard wound care protocols. The technology can be integrated into existing treatment regimens without disrupting established workflows, often improving overall outcomes while reducing treatment duration and complications.
Conclusion
Wound healing technology has reached a revolutionary milestone with the introduction of cold atmospheric plasma therapy. This innovative approach addresses the complex challenges of wound healing through multiple therapeutic mechanisms, offering hope for patients with chronic wounds and healthcare providers seeking effective treatment solutions.
The clinical evidence supporting cold plasma in wound healing continues to grow, with studies demonstrating significant improvements in healing rates, reduced infection, and enhanced patient outcomes. The Mirari Cold Plasma system represents the cutting edge of this technology, utilizing nitric oxide-enriched plasma to deliver targeted, effective wound healing therapy.
As research advances and clinical adoption expands, cold plasma therapy promises to become an integral component of comprehensive wound care protocols. The technology’s safety profile, effectiveness across diverse wound types, and compatibility with existing treatments position it as a transformative force in modern wound healing practice.
Healthcare providers and patients alike benefit from cold plasma’s non-invasive approach, rapid results, and sustained healing improvements. As we continue to understand the full potential of this technology, cold plasma therapy will undoubtedly play an increasingly important role in advancing wound healing outcomes and improving quality of life for patients worldwide.
References
- Cert Vohra Wound Care. (2024). Four Stages of Wound Healing.//cert.vohrawoundcare.com/the-four-stages-of-wound-healing-an-updated-overview-for-clinicians/
- PMC. (2022). Innovative Treatment Strategies to Accelerate Wound Healing.//pmc.ncbi.nlm.nih.gov/articles/PMC9367945/
- PMC. (2023). The Role of Cold Atmospheric Plasma in Wound Healing.//pmc.ncbi.nlm.nih.gov/articles/PMC10219374/
- PMC. (2023). The Regulatory Mechanism of Cold Plasma in Relation to Cell Activity.//pmc.ncbi.nlm.nih.gov/articles/PMC10138629/
- Mirari Doctor. (2025). Wound Healing – A Complete Guide to Mirari Cold Plasma./wound-healing/
- PMC. (2023). Cold Plasma Therapy in Chronic Wounds—A Multicenter Study.//pmc.ncbi.nlm.nih.gov/articles/PMC10419810/
- Mirari Doctor. (2025). Cold Plasma and Its Role in Wound Healing./cold-plasma-in-wound-healing/
- Mirari Doctor. (2024). Is Cold Plasma FDA Approved?/is-cold-plasma-fda-approved/
- NCBI Bookshelf. (2025). Wound Healing Phases.//www.ncbi.nlm.nih.gov/books/NBK470443/
- Medizin Online. (2021). Cold plasma promotes wound healing in diabetic foot ulcers.//medizinonline.com/en/cold-plasma-promotes-wound-healing-in-diabetic-foot-ulcers/
- AOT Inc. (2025). Clinical Evidence And Wound Healing Studies.//aotinc.net/clinical-evidence/
- Cambridge Media. (2025). Cold Plasma An emerging technology for clinical use in wound healing.//journals.cambridgemedia.com.au/jwm/volume-25-number-3/cold-plasma-emerging-technology-clinical-use-wound-healing
- Athenaeum Publishing. (2024). Use of Cold Plasma in the Treatment of Infected Wounds.//athenaeumpub.com/use-of-cold-plasma-in-the-treatment-of-infected-wounds/
- Frontiers in Medicine. (2025). Advancing chronic and acute wound healing with cold plasma therapy.//www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2025.1527736/full
- Frontiers in Pharmacology. (2025). Mechanisms underlying the wound healing and tissue regeneration.//www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1574715/full
- ScienceDirect. (2025). Wound healing in the modern era: Emerging research, biomedical innovations.//www.sciencedirect.com/science/article/abs/pii/S1773224725004617
Related articles
Made in USA