Cold plasma in tissue regeneration represents a groundbreaking advancement in medical technology that harnesses the power of ionized gas to accelerate healing processes. This innovative therapy utilizes reactive oxygen and nitrogen species to stimulate cellular repair mechanisms, offering new hope for patients with chronic wounds, surgical recovery needs, and various regenerative medicine applications.
Unlike traditional thermal treatments that can damage healthy tissue, cold atmospheric plasma operates at body temperature while delivering therapeutic benefits through controlled biochemical reactions. The technology has gained significant attention from healthcare professionals worldwide due to its non-invasive nature and remarkable ability to enhance natural healing processes.
Understanding Cold Plasma Technology in Medical Applications
What is Cold Atmospheric Plasma?
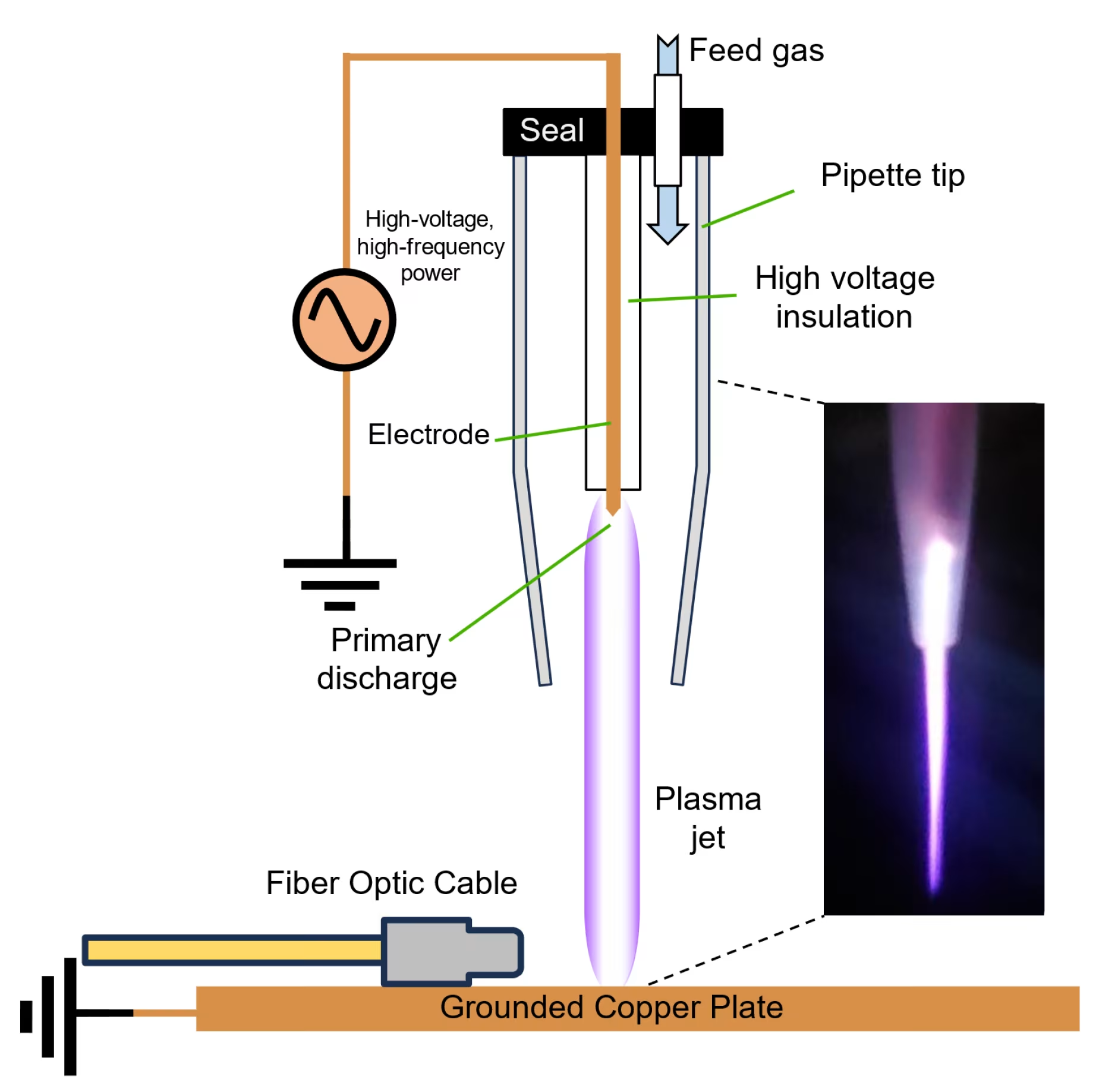
Cold atmospheric plasma (CAP) is the fourth state of matter, created when energy is applied to gas molecules, causing them to become ionized while maintaining temperatures near body temperature. This unique characteristic makes cold plasma in tissue regeneration particularly valuable for medical applications, as it delivers therapeutic effects without causing thermal damage to surrounding healthy tissue.
The plasma contains a complex mixture of reactive species, including reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, electric fields, and electromagnetic radiation[1]. These components work synergistically to create an environment that promotes cellular repair and regeneration.
Scientific Mechanisms Behind Cold Plasma Healing
The therapeutic effects of cold plasma in tissue regeneration occur through multiple interconnected pathways. When applied to biological tissues, the reactive species generated by cold plasma interact with cellular components to trigger beneficial responses.
Research has demonstrated that cold plasma treatment modifies the persistence levels of inflammatory cytokines and growth factors, including IL-1, IL-8, TGF-β, TNF-α, and INF-γ, promoting healing through more rapid initiation of the proliferative phase[1]. This controlled inflammatory response is crucial for effective tissue regeneration.
Nitric Oxide Production and Cellular Signaling
One of the most significant mechanisms involves the production of nitric oxide (NO), which acts as a key signaling molecule in tissue repair. Cold plasma treatment triggers NO production, which enhances cell migration and promotes the assembly of endothelial cells into vessel-like structures essential for wound neovascularization[1].
Studies have shown that cold plasma significantly increases endothelial nitric oxide synthase (eNOS) expression and activation, leading to enhanced NO production that affects neural signaling and promotes angiogenesis[2]. This mechanism is particularly important for patients with compromised circulation or diabetic complications.
Reactive Species and Cellular Activation
The reactive species generated by cold plasma activate various cellular functions critical for tissue regeneration. These include stimulation of neutrophils, macrophages, endothelial cells, keratinocytes, and fibroblasts, which help maintain tissue oxygenation, initiate angiogenesis, and promote collagen synthesis[3].
Clinical Applications of Cold Plasma in Tissue Regeneration
Wound Healing and Chronic Ulcer Treatment
Cold plasma in tissue regeneration has shown remarkable success in treating various types of wounds, particularly chronic ulcers that resist conventional therapies. The technology addresses multiple aspects of wound healing simultaneously, including infection control, inflammation reduction, and cellular stimulation.
Clinical studies have demonstrated that cold plasma treatment significantly accelerates wound healing rates, with particularly pronounced effects in elderly patients and those with compromised healing capacity[4]. The treatment markedly inhibits pro-inflammatory cytokines while promoting beneficial cellular responses.
Diabetic Foot Ulcer Management
Diabetic foot ulcers represent one of the most challenging wound healing scenarios, often complicated by poor circulation, neuropathy, and increased infection risk. Cold plasma therapy has emerged as a promising treatment option for these difficult cases.
Randomized controlled trials have shown that patients with diabetic foot ulcers treated with cold plasma exhibited faster wound epithelialization and reduced ulcer size compared to standard treatments[5]. The antimicrobial properties of cold plasma help address bacterial colonization while simultaneously promoting tissue regeneration.
Surgical Recovery and Post-Operative Healing
The application of cold plasma in tissue regeneration extends beyond chronic wound care to include surgical recovery enhancement. Post-operative patients often benefit from accelerated healing times and reduced complication rates when cold plasma therapy is incorporated into their recovery protocols.
Research indicates that cold plasma treatment can improve the quality of newly formed tissue, with treated wounds showing better structural integrity and elasticity compared to conventional healing methods[5]. This enhanced tissue quality translates to improved long-term outcomes for surgical patients.
Technical Specifications and Treatment Parameters
| Parameter | Specification | Clinical Significance |
|---|---|---|
| Operating Temperature | 20-40°C (body temperature) | Eliminates thermal damage risk |
| Treatment Duration | 30 seconds to 15 minutes | Customizable based on condition severity |
| Reactive Species | ROS, RNS, NO production | Multiple therapeutic mechanisms |
| Frequency Range | Variable (device-dependent) | Optimized for tissue penetration |
| Power Output | 2-4W (typical range) | Safe energy delivery levels |
| Application Method | Direct or indirect contact | Flexible treatment approaches |
Advanced Cold Plasma Devices in Clinical Practice
Mirari Cold Plasma System Technology
One of the pioneering medical devices applying cold plasma therapy is the Mirari Cold Plasma System, developed by General Vibronics and brought to market by Mirari Doctor. This innovative device utilizes nitric oxide (NO) instead of traditional ROS-based systems to promote safe, non-thermal healing.
The Mirari system represents a significant advancement in cold plasma technology, offering healthcare providers a portable, user-friendly device that delivers consistent therapeutic outcomes. The device’s unique approach to NO generation provides enhanced safety profiles while maintaining therapeutic efficacy.
Technical Innovation and Safety Features
The Mirari Cold Plasma device incorporates advanced safety features including temperature monitoring, automatic shutoff mechanisms, and precise energy delivery controls. These features ensure that cold plasma in tissue regeneration can be applied safely across various clinical settings.
Healthcare professionals using the Mirari system report improved patient outcomes and reduced treatment times compared to conventional therapies. The device’s portability and ease of use make it particularly valuable for outpatient clinics and home healthcare applications.
Clinical Benefits and Treatment Outcomes
| Application Area | Treatment Duration | Reported Outcomes | Patient Population |
|---|---|---|---|
| Chronic Wounds | 5-15 minutes per session | 70-90% healing improvement | Diabetic, elderly patients |
| Surgical Sites | 2-10 minutes post-op | Reduced infection rates | Post-surgical patients |
| Burn Treatment | 1-5 minutes per area | Accelerated epithelialization | Burn injury patients |
| Pressure Ulcers | 10-20 minutes per session | Enhanced granulation tissue | Immobilized patients |
| Venous Ulcers | 5-15 minutes per treatment | Improved circulation markers | Vascular disease patients |
| Dermatological Conditions | 2-8 minutes per lesion | Reduced inflammation | Various skin conditions |
Safety Considerations and Contraindications
Patient Safety Protocols
While cold plasma in tissue regeneration offers significant therapeutic benefits, proper safety protocols must be followed to ensure optimal outcomes. Healthcare providers should conduct thorough patient assessments before initiating treatment to identify any potential contraindications.
The non-thermal nature of cold plasma significantly reduces safety concerns compared to traditional thermal therapies. However, certain patient populations may require modified treatment protocols or additional monitoring during therapy sessions.
Contraindications and Precautions
Patients with active infections in the treatment area may require preliminary antimicrobial therapy before cold plasma application. Additionally, individuals with certain medical devices or specific medical conditions should be evaluated carefully before treatment initiation.
Pregnant patients and those with compromised immune systems may require specialized treatment protocols or alternative therapeutic approaches. Healthcare providers should always consult current clinical guidelines when treating these populations.
Monitoring Treatment Response
Effective cold plasma therapy requires ongoing monitoring of patient response and treatment outcomes. Healthcare providers should document healing progress, assess for adverse reactions, and adjust treatment parameters as needed to optimize results.
Regular wound assessments, photographic documentation, and patient feedback help ensure that cold plasma in tissue regeneration is delivering expected therapeutic benefits while maintaining patient safety and comfort.
Comparing Cold Plasma to Traditional Therapies
Advantages Over Conventional Treatments
Cold plasma in tissue regeneration offers several distinct advantages over traditional wound care approaches. The technology’s ability to simultaneously address multiple aspects of healing—including infection control, inflammation reduction, and cellular stimulation—sets it apart from single-mechanism therapies.
Unlike antibiotic treatments that may contribute to resistance development, cold plasma provides broad-spectrum antimicrobial effects without promoting bacterial resistance. This characteristic makes it particularly valuable for treating chronic wounds with complex bacterial colonization patterns.
Cost-Effectiveness and Treatment Efficiency
Clinical studies suggest that cold plasma therapy may reduce overall treatment costs by accelerating healing times and reducing the need for multiple interventions. Patients often experience faster recovery with fewer complications, leading to improved quality of life and reduced healthcare utilization.
The non-invasive nature of cold plasma treatment eliminates many risks associated with surgical interventions while providing comparable or superior therapeutic outcomes in many cases.
Future Directions and Research Developments
Emerging Applications in Regenerative Medicine
Research into cold plasma in tissue regeneration continues to expand, with new applications being explored in various medical specialties. Current investigations include applications in dental regeneration, orthopedic healing, and cosmetic dermatology.
Scientists are working to optimize treatment protocols for specific conditions and patient populations, with the goal of maximizing therapeutic benefits while minimizing treatment times and costs. These efforts are supported by growing clinical evidence demonstrating the technology’s versatility and effectiveness.
Integration with Other Therapeutic Modalities
Future developments may include combination therapies that integrate cold plasma with other regenerative medicine approaches, such as stem cell therapy, growth factor applications, and advanced wound dressings. These synergistic approaches could further enhance healing outcomes for challenging cases.
The Mirari Doctor platform at miraridoctor.com continues to support research and development efforts, providing healthcare professionals with access to cutting-edge cold plasma technology and clinical support resources.
Patient-Focused Frequently Asked Questions
How long does it take to see results from cold plasma treatment?
Most patients begin to notice improvements in wound healing within the first few treatment sessions, typically within 1-2 weeks of initiating therapy. However, the timeline for complete healing varies depending on wound size, patient health status, and underlying conditions. Chronic wounds that have been present for months or years may require several weeks of consistent treatment to achieve optimal results.
Healthcare providers typically schedule follow-up appointments every 1-2 weeks to monitor progress and adjust treatment protocols as needed. Patients with diabetic ulcers or other complex conditions may require longer treatment courses but often experience significant improvements in wound quality and healing trajectory.
Is cold plasma treatment painful or uncomfortable?
Cold plasma therapy is generally well-tolerated by most patients, with minimal discomfort during treatment. The technology operates at body temperature, eliminating the burning sensations associated with thermal therapies. Some patients may experience a mild tingling sensation or slight warmth during treatment, but these sensations are typically mild and temporary.
Patients with sensitive skin or acute wounds may experience slightly more sensation during initial treatments, but this typically decreases as healing progresses. Healthcare providers can adjust treatment parameters to ensure patient comfort while maintaining therapeutic effectiveness.
Can cold plasma treatment be used on infected wounds?
Yes, cold plasma therapy is particularly effective for infected wounds due to its powerful antimicrobial properties. The treatment can eliminate a wide range of pathogens, including antibiotic-resistant bacteria, while simultaneously promoting healing processes. However, severe infections may require additional antimicrobial therapy alongside cold plasma treatment.
Healthcare providers typically assess infection severity before initiating treatment and may recommend preliminary infection control measures for heavily contaminated wounds. The broad-spectrum antimicrobial effects of cold plasma make it valuable for preventing reinfection during the healing process.
How often should cold plasma treatments be administered?
Treatment frequency depends on wound characteristics, patient health status, and healing response. Most patients receive treatments 2-3 times per week initially, with frequency adjustments based on healing progress. Acute wounds may require daily treatments for the first week, while chronic wounds typically benefit from consistent twice-weekly sessions.
Healthcare providers monitor healing progress and adjust treatment schedules accordingly. As wounds begin to heal, treatment frequency may be reduced while maintaining therapeutic benefits. The total treatment course typically ranges from 2-8 weeks, depending on individual patient needs.
Are there any side effects or risks associated with cold plasma therapy?
Cold plasma treatment has an excellent safety profile with minimal reported side effects. The most common reactions include temporary mild redness or slight skin irritation at the treatment site, which typically resolves within hours. Unlike thermal therapies, cold plasma does not cause burns or permanent tissue damage when used properly.
Rare side effects may include temporary increased wound drainage as the healing process accelerates, which is generally considered a positive sign of treatment response. Patients with specific medical conditions or those taking certain medications should discuss potential interactions with their healthcare provider before beginning treatment.
Medical Disclaimer: This information is for educational purposes only and should not replace professional medical advice. Always consult with qualified healthcare providers before starting any new treatment. Individual responses to cold plasma therapy may vary, and treatment decisions should be based on comprehensive medical evaluation and professional judgment.
References
- Bolgeo, T., et al. (2023). The Role of Cold Atmospheric Plasma in Wound Healing Processes in Critically Ill Patients. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC10219374/
- Arndt, S., et al. (2019). Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. PubMed. https://pubmed.ncbi.nlm.nih.gov/31265742/
- Murali, R., et al. (2024). Cold atmospheric plasma (CAP) in wound healing. Regenerative Medicine. https://rem.bioscientifica.com/view/journals/rem/2024/1/REM-23-0026.xml
- Zhou, J., et al. (2025). Low-temperature cold plasma promotes wound healing by inhibiting skin inflammation and improving skin microbiome. Frontiers in Bioengineering and Biotechnology. https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1511259/full
- Mirari Doctor. (2025). Cold Plasma and Its Role in Wound Healing. https://miraridoctor.com/cold-plasma-in-wound-healing/
Related articles
Made in USA