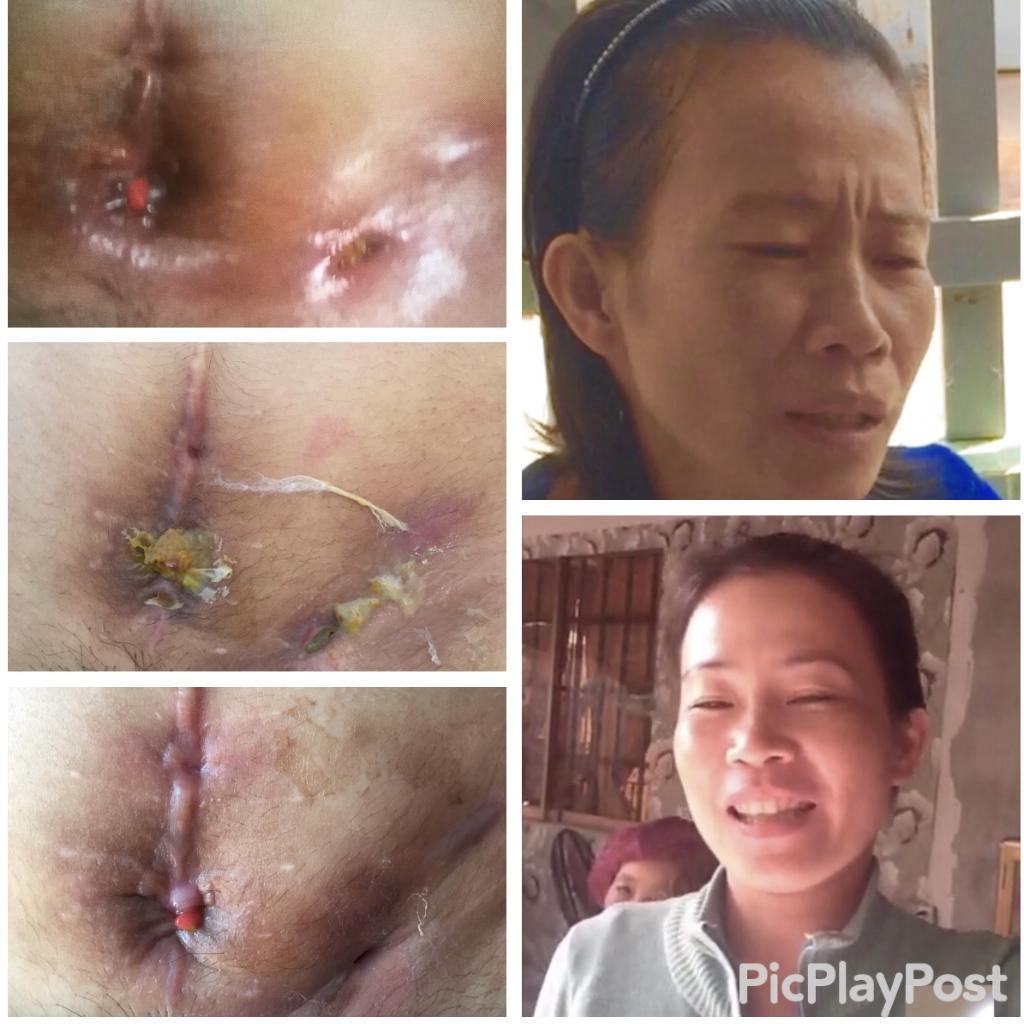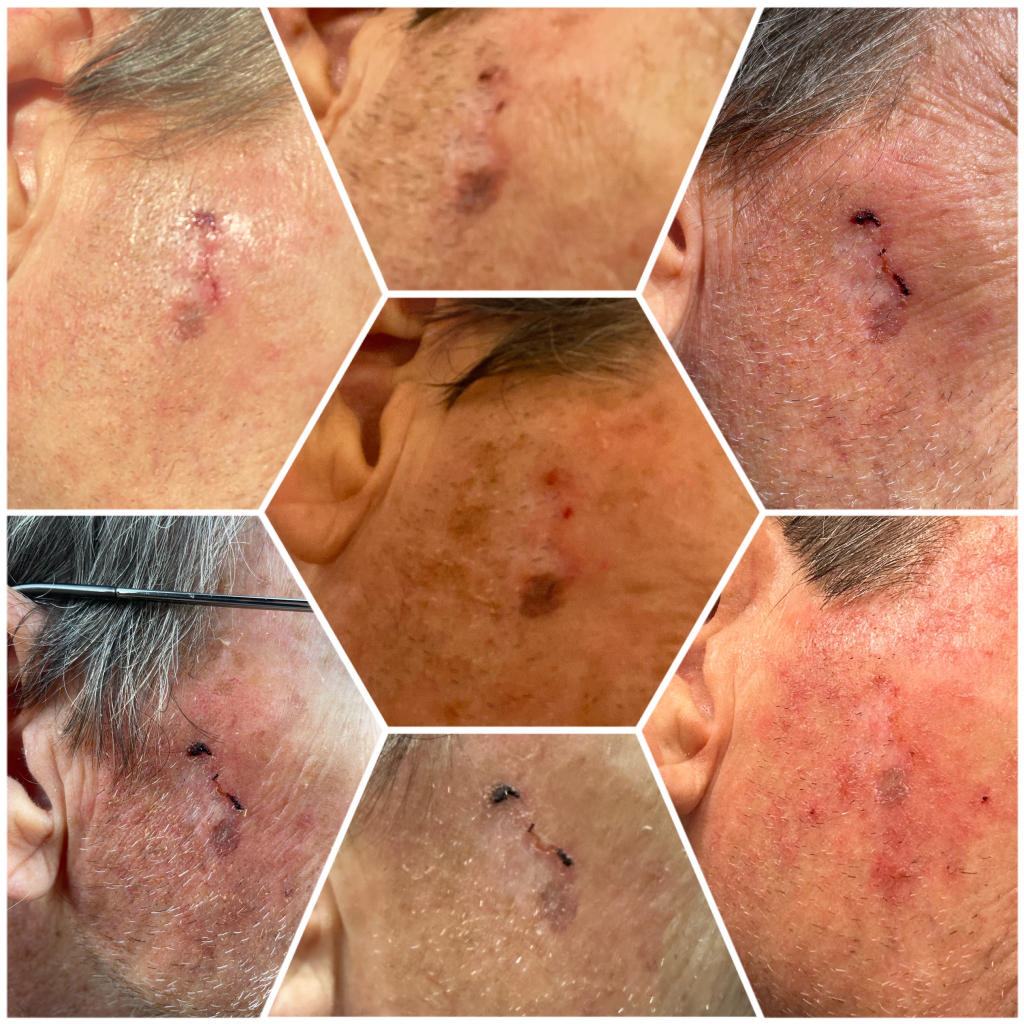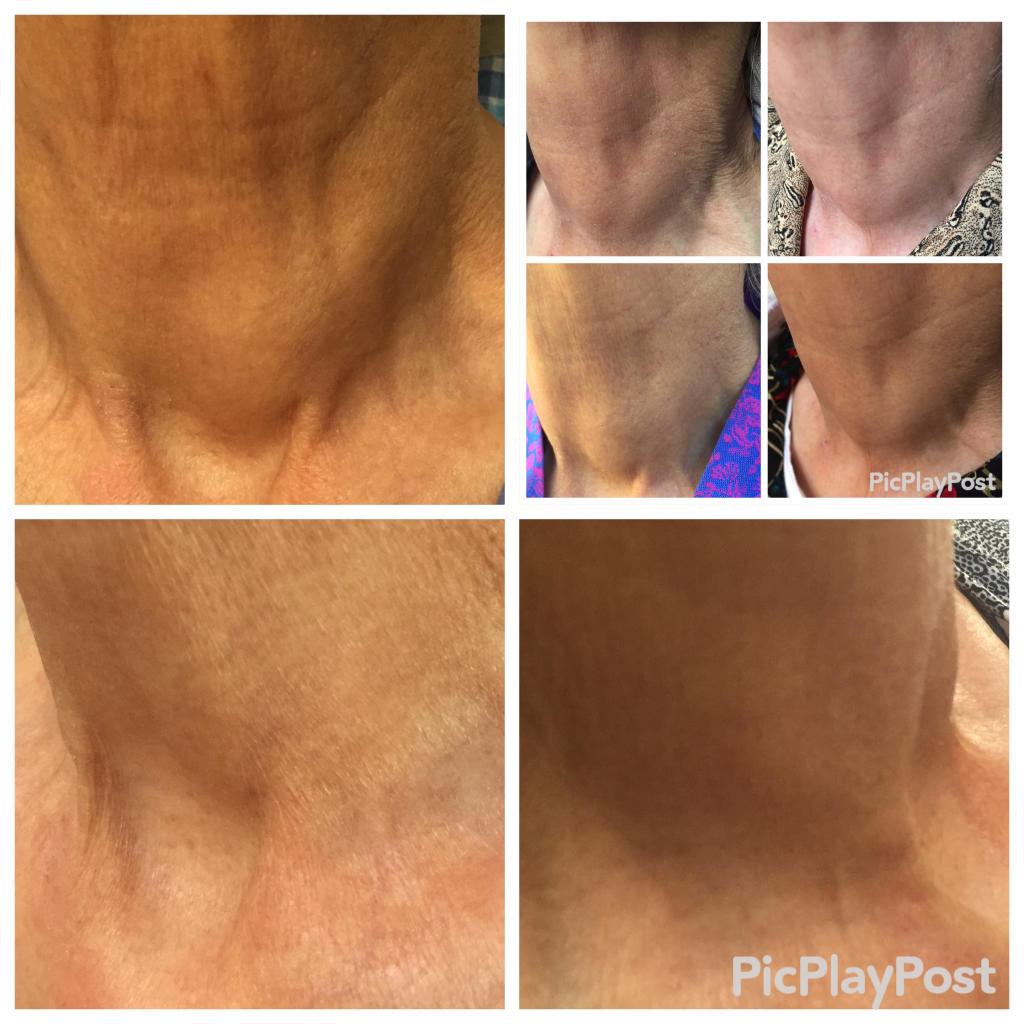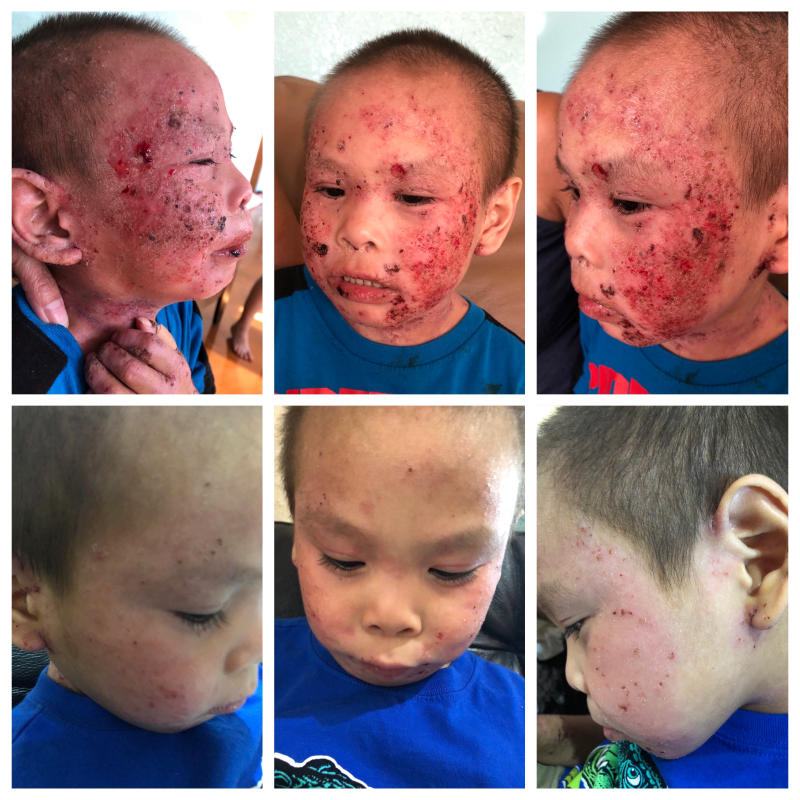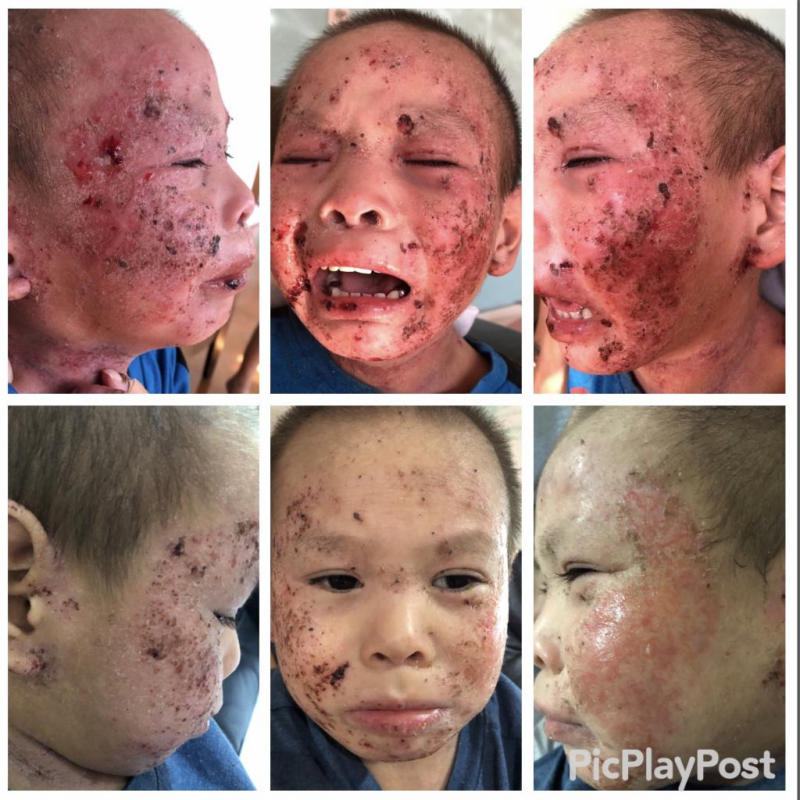

The Mirari Cold Plasma System is approved by the Thai FDA,Vietnam MOH and FDA-Cleared in the United State for specific uses. The testimonials and content presented here are for informational purposes only. Always consult with a healthcare professional before considering any treatment or procedure.
Hệ thống Plasma Lạnh Mirari được phê duyệt bởi Cục Quản lý Thực phẩm và Dược phẩm Thái Lan (Thai FDA), Bộ Y tế Việt Nam (Vietnam MOH) và được FDA Hoa Kỳ cấp phép strong>cho các mục đích sử dụng cụ thể. Các lời chứng thực và nội dung được trình bày ở đây chỉ nhằm mục đích cung cấp thông tin. Luôn tham khảo ý kiến của chuyên gia 1 y tế trước khi cân nhắc bất kỳ phương pháp điều trị hoặc thủ thuật nào
ระบบพลาสมาเย็น Mirari ได้รับการอนุมัติจาก Thai FDA, Vietnam MOH และ FDA-Cleared ในสหรัฐอเมริกา สำหรับการใช้งานเฉพาะ คำรับรองและเนื้อหาที่นำเสนอที่นี่มีวัตถุประสงค์เพื่อให้ข้อมูลเท่านั้น ปรึกษาผู้เชี่ยวชาญด้านการดูแลสุขภาพเสมอก่อนพิจารณาการรักษาหรือขั้นตอนใดๆ
The Mirari Cold Plasma System is approved by the Thai and Vietnam FDA for specific uses. For viewers outside of Thailand and Vietnam, the device is not currently available for sale or use. The testimonials and content presented here are for informational purposes only. Always consult with a healthcare professional before considering any treatment or procedure. Join our $5 monthly subscription to participate in monthly Q&A sessions about Mirari and be on a priority waiting list for when the device becomes available in your country. Your subscription helps prioritize the Mirari rollout globally.
免疫治疗是医学领域快速发展的方向,旨在激活或调节免疫系统,以对抗包括癌症和自身免疫性疾病在内的多种疾病。通过增强或引导免疫反应,免疫治疗可实现多种治疗目标。Mirari 冷等离子体治疗作为一项创新技术,已展现出在辅助免疫治疗方面的潜力。其冷等离子体所具备的独特物理与化学特性,有助于激活 T 细胞与 B 细胞的功能,从而增强机体的免疫反应,提升治疗效果。